The Outbreak of Canine Parvovirus in North America
Introduction
By Gwen Tosaris
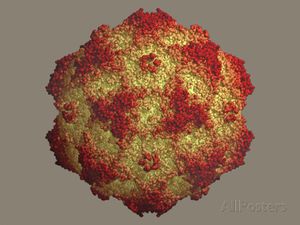
The Canine Parvovirus (CPV) is a deadly viral disease that leads to the enteric infection of canines via direct contact. This highly contagious pathogen has the ability to spread within 3 to 7 days to dogs in close vicinities. The viral pathogen enters the body orally and attaches itself to the villi in the gastrointestinal tract, preventing the proliferation of the host's cells. The dying cells leads to the deterioration of the gastrointestinal tract. The effect of this enteric pathogen leads to severe dehydration, susceptibility to bacterial infections, and internal bleeding. Traveling between hosts through fecal contact, CPV outbreaks are common among canines within a close range. CPV outbreaks have been noted globally. In North America, recent outbreaks of this virus in St. Kitts and Alaska leads to the question of how determining the root of the outbreak can aid in research and prevention of the deadly disease 10].
Structure and Significance
The canine parvovirus strain 2 (CPV-2), or “Parvo,” is a single-stranded DNA virus, non-enveloped, that leads to a deadly enteric infection in canines. Outbreaks have been reported globally - this virus has been sighted in New Zealand, Australia, Asia, Europe, North America, and the Caribbean - and it kills thousands of dogs each year [1]. The virus first surfaced in Europe during the late 1970s. The disease reached epidemic proportions in 1978 that lasted until 1979 when a vaccine was discovered. The virus was first identified with the aid of electron microscopy. Scientists named it CPV-2 as it held close relation to CPV-1 — a viral disease that is now placed in an entirely different category. Canine parvovirus, group II, belongs to the Parvovirus genus and the Parvoviridae family. Its higher classification is the Protoparvovirus [2].
CPV-2 infections are virulent and may cause gastroenteritis (hemorrhagic enteritis, or hemorrhaging due to inflammation of the intestines), myocarditis (inflammation of the heart), and lymphopenia (low levels of lymphocytes) in canines, among several other diseases systemically [3]. Three structural proteins, VP1, VP2, and VP3, along with NS1 and NS2, two non-structural proteins, assemble the parvovirus (Figure 1[12]). Together the proteins form an icosahedral virus, meaning that the virus contains identical subunits to form a symmetrical, equilateral triangle [1]. The VP2 protein is a main structural component of CPV-2 and maintains an eight-stranded, antiparallel β-barrel. The remainder of the VP2 protein is composed of loops that attach to the β-barrel [1]. This strain originated from Feline Panleukopenia (FPLV), also known as “Feline Distemper,” a contagious and fatal disease affecting felines [4]. FPLV, similarly to CPV-2, is known for attacking and spreading in rapidly dividing cells [4]. FPLV is not as common due to the widespread use of a preventative vaccine [5]. Emerging from two or more mutations of FPLV, CPV-2 virus infect canine hosts.
Current efforts to study the effects of canine parvovirus are aimed at the development of preventative vaccines. Understanding the evolution and developments of this disease may also lead to other initiatives in the protection and prevention of outbreaks. In additon, studying the disease raises awareness, which helps keep canine populations safe from exposure. Although there are no reported cases of the disease in humans, variants of this disease may one day mutate to the point of allowing human infection, further substantiating the importance of studying CPV and its variant strains. Canine parvovirus is a deadly disease that deserves intense research to keep animals and humans safe.
Symptoms and Mode of Transmission
The disease has an extremely high mortality rate. Its rate is higher in puppies (91%), ranging from six to twenty weeks old, than adult dogs (10%), as well as dogs who have not been vaccinated with the Distemper-Parvo vaccine, otherwise known as DA2PV [6]. Puppies who come into contact with the CPV-2 are at high risk for heart failure and myocarditis. Other mammals susceptible to CPV include raccoons, coyotes and wolves.
The virus can resist disinfectants as well as pH and temperature variations, making this highly contagious virus easy to transmit [3]. Furthermore, the virus can thrive indoors and outdoors, though it has more difficulty in outdoor environments [3]. CPV-2 is most prevalent in dog and animal shelters where animals are in close vicinity to one another. It can be found in fecal matter as well on other surfaces such as water bowls and dog collars [1].
The acute symptoms of CPV-2 develop within 3 to 7 days of being contracted. Entering the canine’s body orally or nasally, the virus first attacks the lymph nodes [6]. Incubating for two days there, the virus reproduces in lymphocytes. Lymphocytes, white blood cells maintaining a round nucleus, are part of the lymphatic system. Since the lymphatic system aids in the circulation of blood throughout the body, deterioration of this system is immediately detrimental to the affect animal. Neutropenia, which is the lack of neutrophils in the body, will also result from the production of the affected lymphocytes. While some of these lymphocytes begin to die, known as lymphopenia, others travel through the bloodstream with the parvovirus [6]. The virus may then continue to spread to newer, healthier blood cells — among other rapidly dividing cells — within the body.
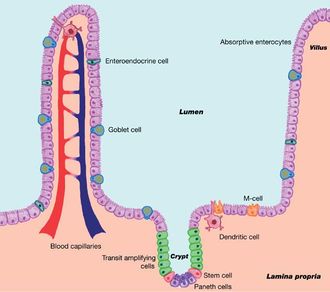
Eventually reaching the gastrointestinal tract, the virus attaches itself to the villi and continues replication of itself on the epithelial intestinal lining. The invasion and rapid replication of CPV-2 deprives the gastrointestinal tract of nutrients and the intestines' ability to prevent fluid leakage is comrpomised [6]. Within the small and large intestine, the smooth muscle surrounding the lumen is lined with folds or grooves that hold the intestinal glands. The glands, called Lieberkühn or intestinal crypts, allow for the essential, rapid creation of new cells [6]. The virus, invading the crypts, interrupts the body’s ability to replace the old cells in the crypts; this may lead to mesenteric lymph nodes, crypt architectural deterioration, or crypt necrosis (crypt death) (Figure 2[13]) [3]. Failure to absorb proper nutrients and prevent fluid loss also leads to the spread of the gut microbiome. These three things ultimately damage the intestinal surface to such an extent that it can no longer function properly. Moreover, necroscopic lesions scar and damage the intestinal surface, thickening and discoloring the lining [3]. In addition, CPV-2 leads to myocardial infection, and ultimately myocarditis and cardiac failure, as well as bone marrow degradation [6]. Within the bone marrow, the virus further depletes the white blood cell count, which gives it more of an protective advantage when entering the gastrointestinal tract.
Symptoms of canine parvovirus type 2 have a rapid onset. Within days, the intestinal wall weakens and can take at least five days to begin its regrowth. The period of time without the immune system leads to inflammation, internal bleeding, and lack of digestion. Due to lack of digestion and fluid loss - or hypovolemia - into the stool, severe dehydration is a common symptom of CPV-2. In fact, dehydration is one of the greatest life-threats to canines with parvovirus. Other signs and symptoms include loss of appetite, as well as hematemesis and bouts of foul-smelling diarrhea. Due to hypovolemia, acting lethargic or with an altered mental status is also common in dogs with canine parvovirus [6]. Movement becomes increasingly difficult for canines who have contracted the virus. Secondary bacterial infections are also possible and may lead to sepsis. Electrolyte imbalance, such as a lack of potassium, is also possible. Potassium is an essential electrolyte that enables the heartbeat and is involved in muscle contractions. Cardiac arrest may result from a lack of potassium among other electrolytes.
New Developments in CPV-2
There are several known mutations of the canine parvovirus strain type 2 that have been discovered, including CPV-2a, CPV-2b, and CPV-2c. These strains differ in the amino acid base pairings. CPV-2a is an antigenic variant of CPV-2, and the strain was first discovered in 1979. CPV-2b is another strain that is most likely derived from the CPV-2a variant and is now the most common strain of canine parvovirus in North America [1]. These variants are now more common than CPV-2 [7]. In fact, the CPV-2a and CPV-2b antigenic variant strains became the predominant stains in 1984 [3]. As a result, CPV-2 infections have declined. More recently, another variant known as CPV-2c has mutated from of the CPV-2 strain and has been found in Italy, Australia, Asia as well as fifteen North American states since 2000[1]. The CPV-2c mutation involves amino acid substitution in the VP2 protein that alters the Aspartic acid on the base pairs [7]. Viral shedding of a host’s fecal matter continues until the disease has been cured and is an advantageous way for the virus to expose itself to other hosts. Research suggests that the mutation occurred to better stabilize the virus in outside environments and to adapt itself to the feline species, allowing it to replicate within feline tissues [7].
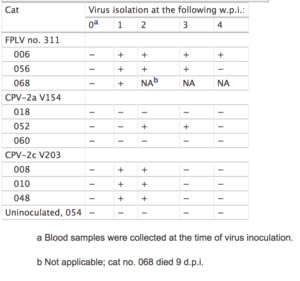
The CPV-2c antigenic variant may not be as virulent as the other variant strains in canines [1]. However, if the CPV-2c variant begins to overtake the CPV-2a and CPV-2b strains, this newer variant may present itself in both canines and felines with the potential to replace FPLV disease in felines (Figure 3[14])[7]. Though CPV-2a and CPV-2b have been observed in some domestic felines in southeast Asia, its pathogenicity is relatively low and does not affect the feline as severely [9]. CPV-2c and FPLV both produce a decrease in white blood cells, or leukopenia, similar to CPV-2 infections [7]. In response, virus-neutralizing (VN) antibodies develop. The amount of VN antibodies is lower in feline test subjects inoculated with CPV-2c compared to felines inoculated with FPLV or CPV-2a (Table 3) [7]. Mild infections of CPV-2c show that the newer variants are increasing in pathogenicity [7]. The main structural protein, VP2, also plays a significant role in in viral-host interactions [11]. This research focuses on the VP2 protein, monitoring it for efficacy and other mutations that strengthen the disease [10].
Diagnosis, Treatment and Immunization
Several tests are available to diagnose a dog with canine parvovirus. The tests may be obtained from rectal swabs that are then analyzed and sequenced to determine the presence of the disease. Though there are several mechanisms for testing, Haemagglutination assays (HA), ELISA assays, Polymerase Chain Reaction (PCR), and Nucleic Acid Sequencing are the most common [1]. Next generation sequencing is another option in diagnosing canines with CPV-2 [10]. The HA test applies fecal samples to red blood cells to observe the presence of the virus. The HA test works by taking the erythrocytes of a species and applying the virus to it. Attaching to the virus, it maintains the ability to form a lattice, thus haemagglutinating [1]. On the blood cells without the virus or with a low concentration of the virus detected, the lattice fails to form. Though relatively simple, the test requires close observation and a high amount of RBCs. On the other hand, Haemagglutination Inhibition assays may also be used by using antibodies to detect the presence of CPV-2.
Currently, PCR is the most common form of diagnostic testing. After being pretreated by boiling the feces, samples extracted are amplified to detect the particles. This method of identification is far more sensitive than HA, making it both easier to use and better equipped to detect canine parvovirus. PCR also has the ability to detect CPV-2 mutants and differentiate them on the gel by using specific primers that work to identify distinct mutants [1]. Rather than use regular PCR, nested PCR is used to better identify the viral replicative form on the gel [1]. This makes it more sensitive than conventional PCR.
The most modern form of sequencing is next generation sequencing. This includes the Illumina (Solexa) Sequencing. The Illumina method is a more advanced or upgraded version of DNA sequencing that aims to read the amplified fragments of DNA. Also called “PCR on a stick,” this process allows for the development of hybridized DNA strands using chips to hold them in. It is a continuous process of denaturaing and synthesizing until the strand is amplified [8]. It is faster and gives a better understanding of the DNA sequences but there is still so much to be revealed about reading the entire DNA sequence together. In one case, researchers used a 500-cycle library kit to sequence the genome. Their first sequences began with a quantity of 10-200ng of RNA that had indexes barcoding the fragments[10]. The parts that are processed are then analyzed using a comparative reference sequence alignment. The reference sequence for parvovirus, NC_001539.1, had multiple reads that are aligned into a table for comparison. Of the rectal samples collected, each genome aligned to the reference genome, 44-96% was read on average[10]. As a whole, less than 1% of the entire metagenome could be read, however.
Once CPV has been diagnosed, supportive care and aggressive therapy work to heal the dog. Due to severe dehydration and hypovolemia as a result of vomiting and diarrhea, fluids are often given in high amounts frequently to adequately hydrate the patient [6]. Blood transfusions are also given to support the low amount of blood cells. Antibiotic drugs are also prescribed to dogs with CPV to help ward off infection.
Immunization for canines decreases the risk of infection even when exposed to the virus. Several brands of vaccinations have been produced, such as DA2PV, a vaccine that targets both CPV and distemper, another viral disease. Some vaccines have begun to target monovalent CPV-2 and are recommended for younger canines [1]. The CPV-2 vaccine has been shown to work against CPV-2a and CPV-2b and is thought to protect against the CPV-2c strain. CPV-2a and CPV-2b vaccinations are now recommended in order to target any mew mutants that are related to the original virus [1]. In North America, veterinarians require this vaccine for dogs of all ages. However, puppies are unable to get the vaccination until they are at least six weeks of age. This is because puppies are must first build up their immune system and screen their B cells and develop other protective mechanisms to fight against pathogens. Immunity is built by their environment but also from their mother’s breast milk, the colostrum. Once the puppies' immune system is sufficiently developed to tolerate the virus, the vaccine is given in a series of two booster shots. The vaccine must be given again the following year and then once every three years for the rest of life.
Outbreaks in St. Kitts and Alaska

Developments in the strain are not solely noted in feline species, but also in regions it has not surfaced in before. In late 2017, the Caribbean region reported that several canines had contracted the disease for the first time in that area. The outbreak stems from the area's large and cramped canine population, conditions that are known to raise the risk of contagion [11]. The cases of hemorrhagic gastroenteritis on St. Kitts attracted research on the strains occurring on that particular island [11]. Focusing on the characterization of the strains in the fecal samples, the article “Molecular characterization of canine parvovirus and canine enteric coronavirus in diarrheic dogs on the island of St. Kitts: First report from the Caribbean region” (Navarro, 2017) tested and characterized the increasing prevalence of CPV in Caribbean dog populations. Based upon research using open reading frames that encode the VP2 protein, it seems that the CPV strain is most similar to the CPV-2a variant (Figure 4[15])[11]. The island of St. Kitts found 24% of tested canines (out of 104 fecal samples) to have CPV [11]. Most of the dogs were within three years of age and had not been vaccinated. North America has shown increasing incidence of canine parvovirus that has most likely proliferated due to exposure from South America. In fact, though the CPV-2a strain is more common in Asia, South America and North America have increasing CPV-2b and CPV-2c variant strains. Thus, the predominance of the CPV-2a strain in St. Kitts indicates changing epidemiological factors in South and North America. Variation is one factor that results in outbreaks in North America.

In addition, the CPV-2a strain proliferates in North American locations due to under-vaccinated canines. In 2016, an outbreak of CPV-2a and CPV-2b took place in Alaska. The outbreak, similarly to the Caribbean, may be attributed to large canine populations since dog sports are common in Alaska [10]. In the article “Investigation of a Canine Parvovirus Outbreak using Next Generation Sequencing” (Parker, 2017), researchers reported the use of genome-sequencing to identify the mechanisms behind the outbreak of CPV within two canine communities. Researchers tested whether the outbreak proliferated due to under-vaccinated canines or due to a new development in the canine parvovirus type 2 strain.
The VP2 protein provided insight to the diversity of CPV variants and also served as a base to compare to new adaptations in variants. Researchers studied the VP2 protein in rectal samples, researchers investigated RNA transcripts and performed deep-sequencing to characterize the strains. Performing next-generation sequencing with Illumina Sequencing, (with PCR, serology and genotyping as a reference,) rectal samples were analyzed to identify any active changes of CPV in rectal samples [10]. VP2 gene location in next-generation sequencing showed the two canine communities developed two distinct virus variants, CPV-2a and CPV-2b (Figure 5[1]). However, the rapid replication of the viruses did not suggest a new installment in the strains, but rather, suggested that the disease contaminated other dogs through nearby under-vaccinated dogs [10]. The process of next-generation sequencing determined that, not only are these viruses capable of antigenic variation but that they also maintain the ability to spread easily in places where prevention tactics and treatment are not always as accessible. The outbreaks in North America show that the disease can to infect canine populations through a combination of genomic change and under-vaccinated communities.
Public Health and Conclusion
In identifying the frequency, distribution, and main causes of disease, epidemologic research aims to prevent and control the risk factors that effect the overall health and well-being of humans and animals. CPV is a deadly enteric disease that can easily spread to canines. By researching and understanding the root cause of this disease and its outbreaks, the health and safety of the community may advance and ultimately may even be able to prevent the eventual return of the viral pathogen. Moreover, researching CPV informs the ways to improve public health and safety and can also improve in the overall understanding of medicine and disease control and prevention. Finally, the study of certain aspects of CPV, such as its pathogenesis and outbreak trends, can alter and elucidate the current scientific perspective of the pathological world. By studying outbreaks and epidemics, researchers may also conclude new ways to prevent the transmission of diseases on a national and global scale.
Though CPV is not currently transmissible to humans, maintaining animal health still affects humans with respect to their support in the food and nutrition industry as well as therapeutically in the home. Maintaining animal health not only reduces the risk of exposure to canine parvovirus but also prevents its transmission to other animals that are not properly cared for. By taking domestic animals to the veterinarian, vaccinating them, and establishing baseline health, both the animal and human community are protected and safe. If canine parvovirus contamination is likely in one’s house or yard, it is important to remember that the disease is robust and difficult to dispel. However, bleach is one household item that does work against CPV and can be used to disinfect contaminated areas. Removing and preventing the spread of the contagion is important given the virus' rapid evolution; the canine parvovirus and variants of this disease may one day be transmissible to the human species as a zoonotic disease. Thus, the effort to maintain a healthy space remains essential. Both individually and communally, precautions must be taken to cure and prevent the propagation of canine parvovirus.
It is important to address public health concerns in North America as well as on an international scale. Though North America continues to progress in the development of medicine and in disease control, there are variables that prevent complete isolation of diseases. In areas with fewer resources available, maintaining a healthy environment becomes increasingly difficult. Thus, dangerous pathogens continue to circulate and play a role in the epidemics and outbreaks that can extend across borders and on a global scale. By working to improve and advance in vaccines and other cures at affordable costs, preventative care can be possible and may improve the well-being of animals and humans worldwide.
References
[1]
[2]
[3]
[4]
[5]
[6]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
- ↑ Nandi, S., and Manoj Kumar. “Canine Parvovirus: Current Perspective.” Indian Journal of Virology : an Official Organ of Indian Virological Society, Springer-Verlag, June 2010
- ↑ Meunier, P.C., et al. “Pathogenesis of Canine Parvovirus Enteritis: The Importance of Viremia.” Veterinary Pathology, Sage Journals, 1 Jan. 1985.
- ↑ “Canine Parvovirus - Digestive System.” Merck Veterinary Manual, Merck Manual
- ↑ “Distemper in Cats.” PetMD, LLC. 1998-2018.
- ↑ Squires, Richard A. “Overview of Feline Panleukopenia - Generalized Conditions.” Merck Veterinary Manual, Merck Manual.
- ↑ “Canine Parvovirus.” Cornell University College of Veterinary Medicine, 29 Jan. 2018
- ↑ Kazuya, et al. “Pathogenic Potential of Canine Parvovirus Types 2a and 2c in Domestic Cats.” Clinical and Diagnostic Laboratory Immunology, American Society for Microbiology, 1 May 2001
- ↑ Slonczewski, Joan, and John Watkins. Foster. Microbiology: An Evolving Science. New York: W.W. Norton, 2017. Print.
- ↑ Ikeda Y., Mochizuki M., Naito R., Nakamura K., Miyazawa T., Mikami T., Takahashi E., (2000) Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology 278:13–19.
- ↑ Parker, Jayme, et al. “Investigation of a Canine Parvovirus Outbreak Using Next Generation Sequencing.” Nature News, Nature Publishing Group, 29 Aug. 2017.
- ↑ Navarro, Ryan, et al. “Molecular Characterization of Canine Parvovirus and Canine Enteric Coronavirus in Diarrheic Dogs on the Island of St. Kitts: First Report from the Caribbean Region.” Virus Research, Elsevier, 26 Aug. 2017.
- ↑ “Parvo Symptoms, Treatment & Prevention.” Organic Pet Digest, OPD.
- ↑ Representation of Intestinal Epithelium.” ResearchGate, Research Gate, 2017.
- ↑ Nakamura, Kazuya, et al. “American Society for MicrobiologyClinical and Vaccine Immunology.” Clinical and Vaccine Immunology, 1 May 2001.
- ↑ Parker, Jayme, et al. “Investigation of a Canine Parvovirus Outbreak Using Next Generation Sequencing Figure 2.” Nature News, Nature Publishing Group Aug. 2017.
