The Skin Microbiome and Malaria
Introduction
By Aldis Petriceks
Malaria is a deadly disease caused by the protozoan Plasmodium, and spread through mosquito bites. [1] The most common ailments are fever, chills, and flu-like symptoms which typically begin within a month of infection (Figure 1). [2] Other symptoms include headache, vomiting, jaundice, shivering, and joint pain. The most notable sign of a malaria infection is paroxysm – which includes cycles of cold and shivering followed by fever and sweating. While immediate treatment can typically facilitate a full recovery, those without access to medical care can die from the disease within days. There are currently 3.4 billion people living in high-risk areas, which led to 500,000 deaths from malaria in 2013 alone. The majority of these deaths occur in underdeveloped tropical and sub-tropical countries, which in turn incur the bulk of the $12 billion USD yearly cost associated with fighting the disease. As a result of malaria's drastic effects, research regarding predictors of the disease is currently at a premium. Such research has recently indicated that mosquito olfaction and the human skin microbiome may play a key role in the incidence of malaria. Specifically, odors produced by skin bacteria have been found to attract anthropophilic mosquitoes, including those which carry diseases such as malaria.
Malaria progresses in two stages. In the first stage, the Plasmodium parasite (in sporozoite form) is injected into the host bloodstream by a mosquito vector, where sporozoites then travel to the liver. [3] Once inside liver cells, these sporozoites spend the next 5-16 days dividing at rapid rates into haploid merozoites. Merozoite build-up in the liver cells causes cell rupture, which then releases the merozoites back into the bloodstream. This begins the second stage of the disease, where the parasites infect red blood cells, undergo many cycles of asexual reproduction, and cause their new host cells to rupture as well, further propagating merozoite production. Infection and rupture cycles repeat every 1-3 days, which manifest themselves in the shivering and fever cycles mentioned above. [3]
While the most commonly-known risk factors for malaria are geographic location, socioeconomic level, and lack of proper medical care, research suggests that an individual’s skin microbiome can heavily influence attractiveness to malaria-carrying mosquitoes. [4] Odorous chemical cues are key to mosquito orientation and landing during host location, and skin flora play a large role in the process by affecting human odor production. Specifically, these microbes metabolize lipids and other compounds in naturally odorless sweat, producing volatile odor compounds attractive to anthropophilic mosquitoes. [5] As such, composition of skin flora can have large effects on host location in such mosquitoes.
The Skin as an Ecosystem
The skin is the largest organ in the body, and constitutes over 1.8 square meters of surface area in which microbes can form habitats (Figure 2). [6] Bacteria, fungi, viruses, and microbial eukaryotes are all common inhabitants of this interface with the outside environment. Similar to any ecosystem, the skin microbiome contains symbiotic organisms of many different natures – many are harmless, others are beneficial to human health, and some are pathogenic and facilitate disease. [6]
The overall environment of the human skin is typically cool in temperature and slightly acidic. [7] However, taking into account the many regions of folds, invaginations, and hair, specific habitats can vary widely between regions of the body. This is especially true in and around the sweat glands. For example, sebaceous glands promote growth of facultative anaerobes such as Propionibacterium acnes due to their oxygen-poor environment (Figure 3). [7]
Relative humidity, temperature, and light also varies widely among body regions. Unsurprisingly, these effects can exert great influence on bacterial composition. The skin microbe S. aureus flourishes in the axillary and groin regions, both of which are high humidity and temperature, and low in light exposure. [8] As such, S. aureus is able to grow in high proportions in these environments. On the other hand, most bacterial strains are not able to grow as successfully in dry and open regions such as the arms and legs.
Considering the influence of topography, temperature, humidity, light, sweat gland, and washing, it is difficult to describe a single, overarching microbiome within an individual’s skin. Rather, the skin microbiome can be thought of as several smaller ecosystems, each of which applies its own specific selection pressures. For example, the scalp is high in proportions of sebaceous glands, which strongly selects for facultative anaerobes and lipophiles such as Propionibacterium. [7] This strong selection pressure results in a relatively low species diversity around the sebaceous glands. In moist regions such as the gluteal crease or sole of the foot, Staphylococcus and Corynebacterium tend to dominate, as they are adept at metabolizing the lipids and proteins present in apocrine sweat.
While dry, desiccated skin such as that of the arms or legs tends to support less-abundant microbial life than warm, moist regions, these areas provide the greatest levels of bacterial diversity throughout the human skin microbiome. One hypothesis for this phenomenon is that the lack of heat and humidity prevents any single bacterial species from flourishing and thus dominating other species, allowing for smaller proportions of many different bacteria to remain. [8]
Anthropophilic Mosquito Olfaction
Mosquitoes rely heavily on olfaction during orientation and landing. Olfactory receptors are based in the antennae and maxillary palpi, with each site containing receptors for specific odor compounds (Figure 4). [9] Upon contact with such compounds, olfactory cells send electrical signals to higher-processing centers in the olfactory system. [10] In anthropophilic mosquitoes, these cues typically come in the form of sweat metabolites, which are given their odor through bacterial metabolism. Studies suggest anthropophilic mosquitoes have evolved to recognize sweat metabolites from human skin bacteria, as these insects greatly prefer natural human scents to synthetic blends of similar salt, water, and heat composition. [11] As variation exists among individuals’ skin flora compositions, relative attractiveness to mosquitoes also differs between humans.
Short-chain carboxylic acids, thioalcohols, benzaldehyde, and other compounds are particularly attractive to anthropophilic mosquitoes. [11] These compounds are common byproducts of volatile-producing skin bacteria, and thus signify nearby presence of a human host. Studies suggest that compounds which are unique to human scent are more attractive to these mosquitoes than more-commonly found odor compounds. [12]
Specific genes have been found to code for olfaction of human sweat odorants in anthropophilic mosquitoes. For example, the AgOr1 gene codes for mosquito sensory identification of the odorant 4-methylphenol. AgOr2 codes for olfaction of 2-methylphenol, a less-common metabolite of human skin flora. It is worth noting that such genes are expressed almost exclusively in female mosquitoes, and transcription of these genes decreases significantly after a blood meal. [12]
Regardless of specific olfactory genes, mosquitoes can typically detect human odor molecules at short ranges for several weeks after production. [13] Anthropophilic species such as the malarial vector Anophiles gambiae Giles sensu stricto prefer human odor compounds over those of other vertebrate species. On the other hand, species such as An. quadriannulatus are preferential towards the scent of cows and other non-human animals.
Microbial Production of Body Odor
Aerobic coryneforms such as Micrococcaceae and Propionibacterium are the most abundant volatile-producers in high-odor regions such as the underarm. [8] Odor intensity typically increases with coryneform abundance and density, more so than in other skin epithelium bacteria such as Staphylococcus (Figure 5). However, there is still debate as to which bacteria exhibit the strongest association with mosquito attraction.
Attractive bacteria produce volatile sweat odors by metabolizing lipids and other compounds present in human sweat. [14] Byproducts of this metabolism such as propionic acid, thioalcohols, and benzaldehyde are highly aromatic and give sweat its characteristic, unpleasant smell. Propionic acid, for example, is a product of amino acid breakdown by Propionibacterium. Isovaleric acid is a common metabolic byproduct of the commensal Staphylococcus epidermidis. [14]
Because volatile odor production is dependent on the specific metabolic pathways of skin epithelial bacteria, different skin flora compositions can lead to varying odor emanations from the human body. Odor production of individual bacterial strains depend largely on the strain’s abundance and capacity to metabolize fatty acids in human sweat. Aerobic coryneforms typically have a high correlation with volatile odor production in humans, owing to their high abundance near sweat glands, and ability to metabolize lipids into free carboxylic acids. [15]
Volatile-producing bacteria contain specific genes associated with production of thioalcohols, short-chain carboxylic acids and other volatile compounds. These genes often code for enzymes such as lipases, which metabolize lipids and fatty acids by breaking them down into smaller, more volatile compounds. [16] Interestingly, volatile compound production may vary between growth phases within an individual bacterial species. For example, volatiles produced by skin epithelial bacteria in stationary phase were found to be more attractive to mosquitoes than those produced in log phase growth. While a partial explanation for this pertains to increased bacterial populations during stationary phase, analysis also suggests that the volatile compounds themselves can also vary between growth phases. [16]
Although the large majority of volatiles produced by skin bacteria originate from the metabolism of lipids, breakdown of aliphatic amino acids can also produce malodorous compounds. [17] Volatile compounds produced through this metabolic pathway are typically the same as, or similar to, compounds produced by lipid catabolism. These include short-chain carboxylic acids such as 3-methylbutanoic acid or propanoic acid.
Anthropophilic Mosquito-Attracting Bacteria
Considering that a bacterium’s pathways for lipid catabolism are key to the specific odor produced from sweat, it is no surprise that mosquito location is dependent on the presence of specific volatile-producing bacteria. Individuals with higher proportions of these attractive bacteria can incur increased mosquito location and biting (Figure 6).[16] For example, Figure 6 depicts several strains of prevalent skin microbes and their relative correlations with mosquito landing (top-right strains are highly attractive, bottom-left are poorly attractive). According to these data, Staphylococcus, Acidobacteria, Delftia, Leptotrichia, and Propionibacteria are all "highly attractive" strains to anthropophilic bacteria. This attractiveness has little to do with the bacteria themselves, but rather the compounds they produce. This notion is supported by the observation that such compounds are sufficient for attraction, as mosquitoes will preferentially land on agar plates coated with them.
Certain compounds may be attractive to mosquitoes because they are produced by vertebrate-specific skin bacteria. [18] For example, Corynebacterium minutissimum is a common colonizer of human skin, yet is uncommon elsewhere. As expected, the sweat metabolites produced by this bacterium are highly attractive to anthropophilic mosquito species.
Anophiles gambiae Giles sensu stricto, one of the main vectors for Malaria in sub-Saharan Africa, has shown differential attraction to several different volatile compounds produced by skin flora.[18] When exposed to individual human scents (in used socks), An. gambiae will prioritize those derived from humans with high proportions of Acidobacteria, Delftia, and Leptotrichia. In addition, increased overall bacterial abundance correlated with increased attractiveness to An. gambiae. On the other hand, bacterial diversity is negatively correlated with An. gambiae attraction.
While volatiles produced by Staphylococcus, Acidobacteria, Delftia, Leptotrichia, and Propionibacteria are highly attractive to anthropophilic mosquito species, those produced by Pseudomonas and Variovorax bacteria are relatively unattractive (Figure 6).[18] Studies suggest that these poorly-attractive bacteria might actually decrease mosquito host location through a number of mechanisms. These include metabolism of volatile compounds produced by attractive bacteria, inhibitory signaling to volatile-producing bacteria, production of compounds which actively repel mosquitoes, and masking of volatile compounds present on the human skin. [16] This may explain why increased bacterial diversity is negatively correlated with An. gambiae attraction: Increased heterogeneity allows for greater proportions of poorly attractive bacteria, which counteract volatile production of attractive bacteria and inhibit mosquito host location. An analogous example is the role of gut bacteria in the human immune system. As such, these beneficial skin bacteria can be thought of as a “built-in defense system” against anthropophilic mosquito location.
Identification and Characteristics of Mosquito-Attracting Bacteria
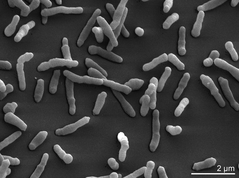
Aerobic coryneforms (one of the most attractive groups of bacteria to anthropophilic mosquitoes) are Gram-positive, rod-shaped microbes (Figure 7). This taxa includes many bacterial genera, including Bacillus, Lactobacillus, Kurthia, Listeria, and Erisypelothrix.[19] While coryneforms are wide-ranging in their metabolism, those that produce volatile odors on human skin are typically classified as lipophilic coryneforms. These bacteria live in and around sweat glands in order to remain close to these sources of lipids and fatty acids. Dermabacter and Propionibacterium are two prominent lipophilic skin coryneforms, and both associated with high attractiveness to anthropophilic mosquitoes.[19]
Staphylococci, another major group of mosquito-attracting bacteria, are spherical, Gram-positive, and organize their cells into clusters (Figure 8). This genus is composed of several well-known skin microbes, such as S. epidermidis, S. aureus, and S. haemolyticus. [20] While odorous compounds produced by Staphylococci are not typically as volatile as those of coryneforms, large numbers of these bacteria on human skin tend to increase mosquito sensation of produced odors. [19]
Factors Affecting the Skin Microbiome
Many factors, both genetic and environmental, combine to influence the volatiles produced by sweat-metabolizing skin microbes. In particular, genetic influences are especially important for skin flora composition. For example, dogs often have significant difficulty in distinguishing between the odors of identical twins, even those raised in separate families.[21] It is also likely for genetic reasons that human individuals tend to show preference for human odors more different from themselves.
One way in which genetics can manipulate human odor emanation is by affecting the composition of sweat itself. Those who produce fewer odor precursors in their sweat are less likely to attract mosquitoes, because their skin flora cannot produce volatile compounds in as great numbers. For example, less than two percent of the human population secretes apocrine and sebaceous sweat which completely lacks odor-precursor lipids and amino acids, and thus their skin bacteria do not metabolize such compounds into volatile forms. Absence of the ABCC11 appears to be the cause of this unique phenotype. [22] Specifically, homozygous genotypes in the SNP (538G>A) tend to result in such odorless sweat, a trait which most commonly manifests itself in Asian populations.
Apart from genetic factors, environmental influences can also serve to manipulate microbial production of volatile compounds. One intuitive cause of augmented volatile production is exercise, which increases perspiration. While most exercise-induced sweating is done through the eccrine glands, augmented exertion is also associated with greater secretion of lipids and oils from apocrine and sebaceous glands. [23] As such, by providing an increased source of lipids and fatty acids, exercise can induce greater levels of volatile compounds on human skin.
Food consumption may also affect human odor production, and thus attraction to mosquitoes. These effects are typically caused by the presence of certain odorous compounds present in foods. For example, foods which are high in sulfur (such as eggs, broccoli, and cabbage) may cause sulfur-containing compounds to be released in the sweat, causing odor even prior to microbial action. [24] However, because most mosquito species locate human hosts via olfaction of aliphatic carboxylic acids, production of these sulfurous odors may not increase attractiveness to such anthropophilic species.
Use of body soap and deodorant have both be analyzed deeply for their effects on the human skin flora. However, to this point, the effects of such products on skin bacteria are unknown, beyond the fact that they alter skin flora composition. [7]
Variability in the Skin Microbiome
Human skin is host to a large number and variety of bacterial species. The hand alone contains about 150 different species, and the inner elbow hosts around 90. [16] Differences in skin flora, however, are typically greater between than within individuals. This is due mainly to genetic factors, which can influence the composition of sweat compounds produced, and thus available to skin bacteria. This interpersonal variation is great enough that individuals can often be identified by bacterial traces left on surfaces such as computer keyboards. Within an individual and body part, skin microbial compositions are typically consistent over time.
An individual’s skin flora composition is heavily influenced by their sweat. Three separate types of glands produce sweat in humans: Eccrine glands, apocrine glands, and sebaceous glands. The eccrine glands produce sweat for thermoregulation, mainly secreting water and salts, with small concentrations of proteins, lipids, and ammonia. [17] As such, regions with high proportions of eccrine cells (such as the hands), typically produce less-volatile sweat than regions with high concentrations of apocrine or sebaceous cells, such as the armpits. Thus, distribution of volatile-producing bacteria on the human body is heavily influenced by distribution of apocrine, eccrine, and sebaceous glands, and in turn influences an individual’s unique odor and selective landing of anthropophilic mosquitoes. These effects are also seen when comparing human skin flora to those of other vertebrates. For example, human sweat contains much larger proportions of triglycerides than the sweat of related primates, and thus yields greater levels of free carboxylic acids from bacterial metabolism.[17] Such differences play a key role in a mosquito’s distinguishing of human hosts from other vertebrates.
Microbe-Targeted Interventions
Because skin flora exert a large influence on human body odor (and thus attractiveness to anthropophilic mosquitoes), interventions have been developed to prevent the spread of malaria and other mosquito-borne disease through inhibited production, or masking of such malodorous compounds. However, bacteria are also key to the health and appearance of human skin, so effective interventions must selectively inhibit the effect of volatile compounds while also leaving the beneficial bacteria unharmed. Naturally-occurring compounds such as citral, citronellal, and geraniol have been identified as safe compounds which inhibit production of volatiles such as isovaleric acid at low concentrations. [25]
Other approaches reduce mosquito attraction by manipulating skin flora compositions, either by increasing proportions of unattractive bacteria (such as Pseudomonas) or decreasing proportions of attractive strains (such Propionibacteria). Surprisingly, many common antiperspirant deodorants are actually counterproductive to this goal, as their active compounds can kill beneficial bacteria, inhibiting their natural anti-volatile effects, and thus also increasing mosquito attraction.[26]
Attractive bacteria and their volatiles may also be used to monitor and/or reduce mosquito populations. By utilizing short-chain carboxylic acid compounds produced by these microbes, mosquitoes could be baited into monitoring or lure-and-kill traps. Long-lasting insecticidal nets are particularly effective (Figure 9).[27] Such approaches have reduced malarial incidence in many African countries. However, cost of production is the main barrier for large-scale integration of these measures throughout the continent.
Malaria can also be prevented through medication. Many malarial medications contain the active ingredient Quinine, which is an alkaloid originating from the Peruvian cinchona tree. Quinine prevents malaria by killing Plasmodium cells before they form into merozoites and infect red blood cells.[28] The molecule accumulates in the food vacuoles of Plasmodium cells, preventing intake of nutrients. The drug can also be used as a post-exposure treatment for people returning from malaria-endemic regions. Chloroquinine is another related and effective antimalarial drug. It is both low-cost and easily manufactured.
Probiotics are also a possible therapy for decreasing preventing malaria through decrease host location. By adding compounds which promote growth of specific microorganisms, such treatments may be able to increase skin flora diversity and decrease proportions of attractive bacteria such as coryneforms. [29]
Future Work
Further research is necessary in order to develop a more cohesive and comprehensive list of mosquito-attracting skin bacteria. It is likely that coryneforms, Staphylococcus, and Acidobacterium will be listed as highly attractive bacteria, although debates regarding these and other possibly-attractive strains are still ongoing. In addition, research regarding microbe-targeted malaria interventions will be a major part of the fight against this disease in the future, as nations attempt to reduce incidence of biting through decreased or masked odor production, as well as odor-baited traps.
References
- ↑ CDC, (2016). "Malaria"
- ↑ Caraballo, H (2014). "Malaria, Dengue, and West Nile Virus"
- ↑ 3.0 3.1 NIAID (2016). "Malaria"
- ↑ Carde, H & Gibson, G (2016). "Host finding by female mosquitoes: mechanisms of orientation to host odours and other cues."
- ↑ Olanga et al (2016). "Attraction of Anopheles gambiae to odour baits augmented with heat and moisture."
- ↑ 6.0 6.1 Grice, E & Segre, J (2016). "The skin microbiome."
- ↑ 7.0 7.1 7.2 7.3 Grice, E & Segre, J (2011) "The skin microbiome."
- ↑ 8.0 8.1 8.2 Rennie et al (1991). "In-vitro and in-vivo studies of human axillary odour and the cutaneous microflora."
- ↑ Takken, W & Knols, B (1999). "Odor-mediated behavior of Afrotropical malaria mosquitoes."
- ↑ Zwiebel, L & Takken, W (1999). "Olfactory regulation of mosquito-host interactions."
- ↑ 11.0 11.1 Olanga et al (2010). "Attraction of Anopheles gambiae to odour baits augmented with heat and moisture."
- ↑ 12.0 12.1 Hallem et al (2004). "Olfaction: mosquito receptor for human-sweat odorant."
- ↑ Zwiebel & Takken (2004). "Olfactory regulation of mosquito-host interactions."
- ↑ 14.0 14.1 Lundstrom & Olsson (2010). "Functional neuronal processing of human body odors."
- ↑ Taylor et al (2003). "Characterization of the microflora of the human axilla."
- ↑ 16.0 16.1 16.2 16.3 16.4 Verhulst et al (2011). "Composition of Human Skin Microbiota Affects Attractiveness to Malaria Mosquitoes."
- ↑ 17.0 17.1 17.2 Smallegange et al (2011). "Sweaty skin: an invitation to bite?"
- ↑ 18.0 18.1 18.2 Verhulst et al (2010). "Differential Attraction of Malaria Mosquitoes to Volatile Blends Produced by Human Skin Bacteria"
- ↑ 19.0 19.1 19.2 Funke et al (1997). "Clinical microbiology of coryneform bacteria."
- ↑ MicrobeWiki. "Staphylococcus."
- ↑ Kuhn (2009). "Body odour of monozygotic human twins: a common pattern of odorant carboxylic acids released by a bacterial aminoacylase from axilla secretions contributing to an inherited body odour type"
- ↑ Preti & Leyden (2010). "Genetic influences on human body odor: from genes to the axillae."
- ↑ American Skin Association. "Healthy Skin."
- ↑ WebMD. "6 Tips for Reducing Body Odor."
- ↑ Ara et al (2006). "Foot odor due to microbial metabolism and its control."
- ↑ Health. "Antiperspirants, But Not Deodorant, May Alter Natural Skin Bacteria in Armpit"
- ↑ Okumu et al (2010). "Development and Field Evaluation of a Synthetic Mosquito Lure That Is More Attractive than Humans"
- ↑ Barennes et al (1996). "Efficacy and pharmacokinetics of a new intrarectal quinine formulation in children with Plasmodium falciparum malaria."
- ↑ NYMAG. "How Probiotics Will Improve Your Skin"
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.