Thermococcus kodakarensis
A Microbial Biorealm page on the genus Thermococcus kodakarensis
Classification
Higher order taxa
cellular organisms; Archaea; Euryarchaeota; Thermococci; Thermococcales; Thermococcaceae; Thermococcus
Genus
Thermococcus kodakarensis
|
NCBI: Taxonomy |
Description and significance
Previously characterized as Pyrococcus sp., Thermococcus kodakarensis is a sulfur-reducing hyperthermophilic archaeon which typically inhabits marine hydrothermal vents and terrestrial hot sulfur springs. This organism and other hyperthermophiles are of great interest as they have evolved mechanisms for adaptation to extremely high temperature environnments. The prokaryote grows at an optimal temperature of 86C, between the ranges of 60-100C, and in a pH range of 5-9. Although this organism is a representation of simple life forms, it grows and thrives in temperatures up to the boiling point of water.(T. Imanaka et al.) In the absence of sulfur, these heterotrophs ferment a variety of organic compounds, including amino acids, peptides, and sugars. Recent accumulation of 16sRNA sequences has indicated the organism belongs to the Thermococcus genus, and not to the originally hypothesized Pyrococcus genus.
Thermococcus kodakarensis was isolated from a solfatara (a volcanic area that releases only hot vapors and sulfurous gases into the environment) on Kodakara Island, Japan, and sequenced by the Kyoto University, Japan.(Morikawa M. et al) This organism produces commercially applicable thermostable DNA polymerases and enzymes that would be useful for such techniques as PCR (Polymerase Chain Reaction).
Thermococcus kodakarensis belongs to the most commonly isolated hyperthermophilic organisms, Thermococcus sp. and are often isolated from marine hydrothermal vents and terrestrial hot sulfur springs. The genome of T. kodakarensis encodes several proteins found within genetic elements similar to those in Pyrococcus spp., implying mechanisms of horizontal gene transfer of mobile elements among the order Thermococcales.
Hyperthermophiles are microorganisms that can grow and survive above 60 C and at an optimum temperature of 80 C. Thermococcus belongs to a group of hyperthermophiles that can grow at extremely high temperature including 100 C (the boiling point of water). (Prieur D. et al) These microorganisms were discovered in 1982 by Stetter and are considered to be the most ancient forms of life. (Adams MW et al) Most hyperthermophiles depend entirely on the reduction of elemental sulfur to hydrogen sulfur for significant growth, resulting in the hindrance of large-scale culture in conventional fermentation systems. (Lepage E et al) After Stetter's first isolation of hyperthermophiles, there have been approximately 20 different genera discovered and added to the class of microorganisms.
Genome structure
The Thermococcus kodakaraensis genome contains 2.09 Million base pairs (bp) and is predicted to have approximately 2357 genes. The average size of a microbial genome is known to be approximately 4 Mb, twice the size of Thermococcus kodakaraensis. The chromosome has a circular topology and the GC content is estimated to be 38 mol%. Seven genes for probable transposases and four virus-related regions are found within the genome. (Fukuri. F et al.)
Cell structure and metabolism
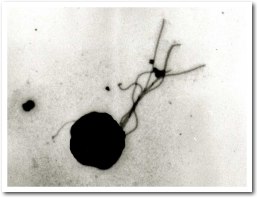
Thermococcus kodakarensis has an irregular cocci (1-2 µm diameter) cell structure and is motile with several polar flagella. T. kodakarensis has a single ether lipid membrane. This strictly anaerobic (existing without oxygen) and sulfur reducing organism uses amino acids, peptides, pyruvate, and starch as its carbon and energy sources. Metabolic pathways of T. kodakarensis include gluconeogenesis and glycolysis and the products of metabolism are hydrogen and hydrogen sulfide gas. (Fukuri. F et al)
Ecology
Thermococcus sp thrive in deep sea hydrothermal vents. The microorganism Thermococcus kodakarensis plays an important role in the ecology of hot-water ecosystems due to the ubiquitous characterisitics exhibited in their habitat. In addition, Thermococcus is the most commonly isolated hyperthermophile, suggesting great abundance throughout the microbial community. (Fukuri F. et al.)
Application to Biotechnology
Thermococcus kodakarensis' attractiveness stems from its ability to survive and grow in extreme environments. The organism's mechanisms of adaptation to such environments has peaked several interests within the biological community. Special proteins of Thermococcus kodakarensis enable it to exhibit some of the specific traits of the genus Thermococcus including additional pyruvate oxidation, nucleotide metabolism, metal ion transporters, improved stress response system, and a distinct restriction system. (Fukuri F. et al) In addition, Thermococcus kodakarensis produces at least three extracellular proteases which exhibit maximum activity at a pH of 7.0 and a temperature of 110 degrees. Further experiments proved these proteases to be thermostable thiol proteases. (M. Morikawa) All enzymes of Thermococcus kodakarensis are found to exhibit remarkable thermostability and are potential resources for procedures such as PCR where enzymes are required to be stable at relatively high temperatures.
Current Research
Hyperthermophilic organisms have become widely studied model microorganisms in many fields. Different fields of study focus on their adaptation to extreme temperatures, phylogeny and genome evolution, molecular deciphering of DNA replication mechanisms in Archaea, and valuable sources of important biotech products (Prieur D. et al)
Kyoritsu College of Pharmacy in Japan has included Thermococcus koadakarensis in a study pertaining to the levels of acetylcholine in biological life forms lacking nervous systems. Acetylcholine is a chemical compound found within the nervous system as a neurotransmitter. They measured levels of acetylcholine using radioimmunoassays (RIA). They sampled acetylcholine levels from organisms of each domain of the phylogenetic tree of life. Thermococcus kodakarensis represented the Archaea domain. The findings of the study indicate ubiquitous expression of acetylcholine has been a "local mediator and modulator of physiological functions since the early beginning of life." (Kawashima K. et al)
The Kyoto University Graduate School of Engineering has studied a type III ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCO) found in Thermococcus kodakarensis. They have found that RuBisCo in Thermococcus kodakarensis does not participate in its classical role in the Calvin-Benson-Bassham cycle, but takes on a new role in adenosine 5'-monophosphate (AMP) metabolism. In this new pathway, the adenine was released from AMP and the phosphoribose moiety entered into central-carbon metabolism. (Sato T et al)
The Kyoto University has also been studying a cell-free protein synthesis system using the lysate of Thermococcus kodakarensis. They had manipulated steps and reagents in hopes of improving temperature range and amount of protein produced from an earlier study using Thermococcus kodakarensis lysate. They succeeded in producing a cell-free system of Thermococcus kodakarensis that has a production potential comparable to that of the Escherichia coli system. (Endoh T. et al)
References
Edited by Jennifer Whitford, student of Rachel Larsen and Kit Pogliano
KMG
