Treatments against Pseudomonas aeruginosa Biofilms in Cystic Fibrosis Patient Lungs
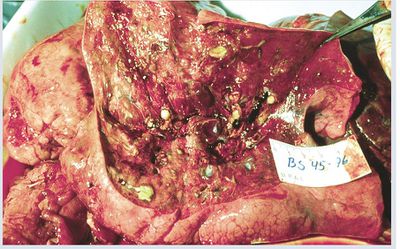
Cystic fibrosis (CF) is the most common autosomal recessive genetic disorder of Caucasians in America [1]. The lifelong struggle of CF patients with Pseudomonas aeruginosa typifies most biofilm infections. The bacteria are ubiquitous and pathogenic for a specific set of compromised individuals. P.aeruginoa, a gram-negative bacterium, thrives in the CF lung environment and is the leading pathogen associated with pulmonary disease in these patients. P. aeruginosa is particularly problematic, infecting the lungs of about 80% of adults with CF [2]. As the bacterial colonies grow, they cause pulmonary problems and eventually premature death. The average life expectancy for people with CF is just 37 years [1]. The infection develops slowly, excluding acute exacerbations, and these temporary stages of relief may be caused by brief antibiotic therapy. The deep-rooted infection cannot yet be cured by conventional antibiotic therapy. The normal course of the infection produces an antibody response to the infecting pathogen, but the antibodies are not effective against sessile (immobible) bacteria. The microcolonies of sessile bacteria in the lung act as sites of origin for spread of the infection.
The predicament facing CF patients is that presently available antibiotics were developed against the planktonic phenotype of P. aeruginosa, and therapeutic agents are chosen on the basis of their efficacy against planktonic cells of this pathogen. Nevertheless, direct observations have shown that the bacteria actually grow in the biofilm phenotype in the lung. Thus, it should come as no surprise that current antibiotic therapies have limited effectiveness in eradicating the biofilm infection.
P. aeruginosa in the CF lung
The CF lung
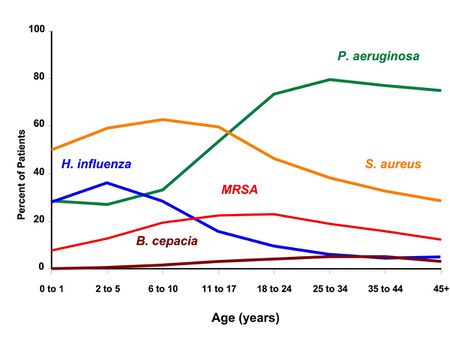
As seen in the figure below, Staphylococcus aureus and Haemophilus influenzae are the most common bacteria isolated from the sputum during the first ten years of life for CF patients, but Pseudomonas aeruginosa is the most prevalent bacteria in the second and third decades of life [3]. The bacterium Methicillin resistant Staphylococcus aureus (MRSA) and Burkholderia cepacia are also present in minor amounts in the CF lung [3]. P. aeruginosa colonizes CF patients in more than 50% of cases [4]. P. aeruginosa, a gram-negative bacterium, is the most frequently described opportunistic pathogen in CF patients [2]. The bacteria are pathogens isolated from CF sputum or bronchoalveolar lavage samples of CF patients of all age groups [1]. Children with CF develop respiratory tract infections early on in their lives [1]. According to the Cystic Fibrosis Foundation in the United States, 29.8% in children from 2-5 years and 81.3% in the age groups of 26-30 years are infected with P. aeruginosa [5]. Colonization of these bacteria in CF airways triggers an inflammatory response with concomitant release of a number of cytokines, such as interleukin-8, which is chemotactic to neutrophils [1]. The buildup of P. aeruginosa in the lungs of CF patients forms biofilms.
P. aeruginosa Biofilms
Biofilms are surface attached communities that can be found in medical, industrial and natural settings. Life in a biofilm most likely represents the predominate mode of growth for microbes in most environments. An extracellular matrix surrounds biofilm microbes; this provides structure and protection to the community. Biofilm-grown bacteria are notable for their resistance to a broad scope of antimicrobial agents including clinically relevant antibiotics. Important research is being done to understand how biofilms form on airway cells and medical implant surfaces. The role of biofilms in host-pathogen interactions and resistance to antibiotic therapy also offers some potential interest. Research on the model organism P. aeruginosa, shows that the unusual intracellular signaling molecule cyclic-di-GMP (c-di-GMP) controls early biofilm formation by regulating both surface motility and exopolysaccharide (EPS) production [6]. These studies are starting to discover the molecular basis of the events required early in the transition to life on a surface.
It was recently revealed that the biofilm regulating factor in P. aeruginosa, c-di-GMP, is a second messenger in bacteria and a central regulator of formation and maintenance of biofilms in a wide variety of organisms [7]. Regulation of c-di-GMP determines whether or not there are biofilms are present in the lungs . Cellular levels of c-di-GMP are controlled through the contrasting activities of diguanylate cyclase (DGC) and phosphodiesterase (PDE)[8]. DGC is a family of enzymes that share the sequence motif, GGDEF domain, and PDE contain a conserved EAL domain [8]. It has been revealed that certain virulence-associated traits are controlled by several PDE’s and DGC’s through changes in the c-di-GMP level [8]. Biofilm formation is enhanced by a DGC dependent increase and reduced by a PDE-mediated decrease in c-di-GMP concentrations [8]. Previous studies revealed that the number of DGC and DGC-PDE proteins affect biofilm regulation in P. aeruginosa. However, it is unknown how the phenotypes of the bacterium are affected by the amount of DGC and PDE proteins [8]. In order to have a strong understanding on the regulation of c-di-GMP, further research must be conducted to create a spectrum of how mutants of P. aeruginosa compare to the wild type phenotypes. A substantial amount of progress has been made in the past decade through the laboratory findings for this disease.
Among the many scientific discoveries, the most recent research involving biofilms has offered the greatest promise to those with CF. Biofilms are the sessile formation of flagella in a cycle involving the second messenger c-di-GMP. A sessile formation of the flagella means that it has lost its tail and is no longer motile. This is significant because biofilm buildups cause the clogging of mucus in the lungs of CF patients. Conducting research on how to break through these sessile formations has created progress towards the development of a cure.
Therapeutic Treatment
Cardiovascular Exercise
Physical exercise is recognized to have considerable clinical benefits for several different disease processes [9] . Exercising when afflicted with CF is challenging because of the shortness of breath one experiences, along with coughing and tiring more easily. Exercise intolerance is a customary characteristic of CF and is reliant on the progression of the disease. Physicians view exercise as having the potential to have a therapeutic effect on the disease, and so it has become a mainstay of the physiotherapy treatment. Exercise programs have the same benefit for people with CF that they have for people without the disease, namely, increasing their fitness and sense of well-being, and undoubtedly improving their overall outlook on life [10]. It is well recognized that vigorous physical activity with deep breathing and coughing promotes airway clearance and helps move mucus out of the lungs. One of the first studies to analyze exercise in Cystic Fibrosis determined that a patient’s fitness level corresponded closely with his or her likelihood to be alive in eight years [5]. The patients with the highest aerobic fitness level had an 83% survival rate for the following eight years, while the middle and lower aerobic levels had 51% and 28% survival predictions comparatively [11]. As the research on exercise has progressed, more physicians are realizing the likelihood of its impact on health and overall well-being.
Extensive research over the past decade has demonstrated that exercise for CF patients can be beneficial to their overall health. In medicine the ability to exercise is related to the capacity of the cardiorespiratory system to transport oxygen to working muscles, and the efficiency of those muscles to extract and use that oxygen. It has been proven and is apparent that maximal oxygen consumption increases with training through physiological adaptions like stroke volume, more oxygen delivery with a lower heart rate, and a more efficient usage of oxygen delivery through biochemical and structural changes to the skeletal muscles [11].
Aerobic fitness is defined as peak oxygen uptake (VO2peak), the highest maximum oxygen uptake attained during testing. This is a pertinent variable in clinical trials studying physical exercise because the VO2peak shows a stronger correlation with the extent of lung damage by P. aeruginosa, as assessed by thin-section chest computed tomography, than a body mass index (BMI) or pulmonary function [12]. The peak oxygen uptake also predicts the progression of CF in terms of chronic inflammation, impaired lung function and even mortality [13]. Previous studies have indicated that adequate aerobic fitness in children with mild to moderately severe CF is associated with a longer time free of hospitalization due to acute respiratory events. There is no demonstrated link that those with a higher VO2peak have less severe illness or whether those patients who remain physically active succeed in delaying the onset of respiratory failure. However, results imply that CF subjects that started with a higher VO2peak lived longer [13].
Hypertonic Saline Solution
The incorporation of exercise into the therapeutic regimen of CF patients has proven to be so beneficial that researchers around the world have investigated the advantages of different types of activity. Such research led to the discovery of how life-changing surfing can be for CF patients. In 2006, Australian researchers realized there was a link between the severity of CF between those who surfed and those who did not. CF patients who surfed had significantly healthier lungs than non-surfing CF patients, and they found that the inhalation of saltwater mist coats the lungs and helps to eliminate the bacteria and mucus. The benefits of surfing prompted the creation of a hypertonic saline (HS) solution to imitate a surf session that could be used daily by CF patients who live inland. Surfing is a great form of exercise and for this specific disease it assists fighting off the harmful effects that occur in the bodies of each patient with CF. One hypothesis regarding the cause of pathogenesis of CF is that the lack of regulation of sodium absorption and chloride secretion instigates the depletion of airway surface liquid, slows mucus clearance, and promotes the formation of adherent mucus plaques on airway surfaces [14]. The mucus plaque plugs obstruct the airway and provide the origin site for the bacterial infections [15].
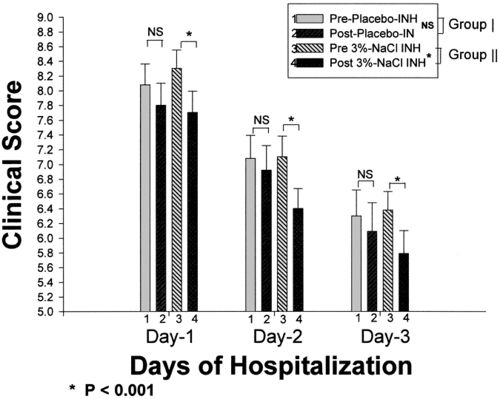
Two teams of researchers working in the United States and Australia identified a simple, inexpensive and effective treatment that increases lung function in CF patients via a HS solution. Through inhalation of a saline solution for ten to fifteen minutes at least twice a day, young patients can avoid a significant part of the damage the disease causes to their lungs. The researchers discovered that the saline solution coats the lungs upon inhalation and thereby restores a layer that coats the airway surfaces, promoting airway clearance of bacteria and mucus. Inhaled hypertonic saline acutely increases mucociliary clearance and, in short-term trials, improves lung function in people with CF [16]. Along with the health benefits, quality of life increased for patients on this treatment: participants in the hypertonic-saline group had significantly fewer days on which they were absent from school or work or unable to participate in other, usual activities, and the mental health domain of the SF-36 was a mean of 5.2 points higher in the hypertonic-saline group than the control group [14].
The incredible effects of surfing prompted researchers to attempt putting the benefits of the ocean into a drug that patients anywhere in the world could use. The Cystic Fibrosis Foundation has funded several studies to determine the mechanisms and effects of HS solution. Elkins’ study using 4mL of 7.0% saline validated the advantages that HS solution provides to the lungs [14]. Reported results stated that the 7.0% HS groups improved their level of lung function, but more importantly saw reductions in the amount exacerbations, antibiotic usage for exacerbations, and absenteeism from school/work or the inability to engage in other activities that were associated with the use of HS.[16]
Antibiotic Treatment

Why Antibiotics Typically Don't Work
Chronic lung infections of P. aeruginosa are the major cause of morbidity and mortality in CF patients [1]. Once formed, these bacterial populations are unmanageable to treat, mainly due to the biofilm mode of growth. This growth in the lungs of CF patients’ accompanies an increased frequency of mutations, as well as the adaption of P. aeruginosa to the inflammatory defense mechanism to the lungs and antibiotic treatments. Mechanisms of resistance such as upregulated efflux pumps, mutations of antibiotic target molecules in the bacteria and chromosomal β-lactamase aid P.aeruginosa survival. These conventional resistance mechanisms and formation of mucoid biofilms, biofilms with alginate as a large part of the polysaccharide protection, allow P. aeruginosa to survive for decades despite the antibiotic therapy, while the lung tissue is gradually destroyed. The immense antibiotic resistance may be attributed to low bacterial metabolic activity and increased doubling times of the bacterial cells in CF lungs.
P. aeruginosa’s ability to form antibiotic resistant biofilms is believed to account for the inability of current therapies to eliminate bacterial infections in the lungs of patients with CF. Although the progression of P. aeruginosa colonization makes eradication essentially impossible, early control seems to delay the onset of chronic lung infection [17]. Different antimicrobial treatment protocols have been established once the first sign of P. aeruginosa colonization is exhibited [17]. The early colonization of these bacteria involves non-mucous colonial morphotypes with low bacterial density. However, once P. aeruginosa begins to colonize, the bacteria density increases and switches to a mucous morphotype with a biofilm mode of growth that is less susceptible to antibiotics [18]. Nebulized colistin, tobramycin, and other antibiotics are already being used to treat P. aeruginosa infections in the CF lung. There is in vitro and in vivo evidence for using joint nebulized and systemic antibiotics in patients with the disease [18].
Promising Antibiotics
Nonetheless, P. aeruginosa may be effectively eradicated during the early stages of colonization with appropriate antibiotic therapy. There is potential prevention of biofilm formation in the CF lung during early stages of colonization. Some evidence exists that suggests early aggressive antibiotic therapy can delay the onset of chronic P. aeruginosa infection in infants or children with CF [19]. However, re-occurrence or re-infection eventually leads to the development of chronic P. aeruginosa infections in teenagers and adults [19].
A recent study done in Spain developed new parameters for defining antibiotic concentrations needed to prevent biofilm formation during early stages of colonization. The P. aeruginosa isolates that were believed to be the early colonizers demonstrated low minimum inhibitory concentration (MIC) to the antibiotics tested. The isolates were completely susceptible to the following antibiotics: tobramycin, ceftazidime, colistin, imipenem, levofloxacin, and ciprofloxacin [20]. Tobramycin, ceftazidime and colistin had the highest percentages of susceptibility [20]. Antibiotics having the lowest biofilm prevention concentration's (BPC) were levofloxacin, tobramycin, colisitin and ciprofloxacin, while the highest were for ceftazidime and imipenem[20]. As expected, antibiotics with a low BPC/MIC ratio had the fastest killing kinetics [20]. These antibiotics include fluroquinolones, aminoglycosides and colisitin. Thus, the antibiotics are active on formed biofilms as seen through their low biofilm inhibitory concentrations (BIC), while also successfully destroying planktonic cells during early biofilm formation [20]. The β-lactam antibiotics like ceftazidime and imipenem, had high BPC/MIC ratios and a small effect on established biofilms [20].
The creation and growth of P. aeruginosa biofilms on mucosal surfaces is the product of complex kinetics. During planktonic dispersal stages of the process, microorganisms are regularly susceptible to antibiotics, however within the biofilms their susceptibility is strongly hindered [20]. Biofilm development may be prevented through antibiotics active on early attached cells or planktonic cells. Results from this study confirm this belief due to usage of P. aeruginosa isolates from initial or early colonization stages that gave low BPCs for fluoroquinolones, tobramycin and colistin. In addition, the results give further evidence in supporting the present rules that recommend CF patients to use these antibiotics in initial or early colonization stages. BPCs are below those of antibiotic concentrations attained at the colonization site, not only when using colistin or tobramycin in aerosols but also oral ciprofloxacin and levofloxacin [14]. Moreover, azithromycin had the lowest BPC/MIC ratio (only 0.11), reflecting additional activity at initial stages of biofilm formation but less so for eradicating well-established biofilms (very high BBC values) [20].
Future Work
There may be hope for biofilm eradication through the usage of iron. Iron is vital to many important physiologic functions in microbial pathogens, such as regulation of gene expression, energy production, and oxygen transport. Iron also promotes biofilm formation on abiotic surfaces, partially regulating surface motility and stabilizing the biofilm polysaccharide matrix. One recent proposed way to eliminate P. aeruginosa biofilm formation is through chelating iron.
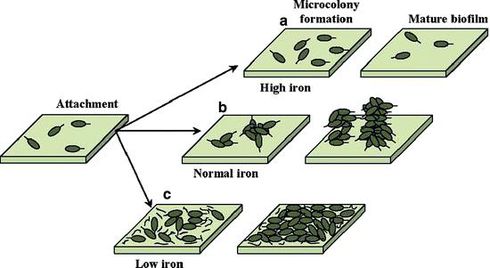
Compounds like lactoferrin, EDTA, conalbumin and gallium have demonstrated a prevention or disruption of biofilm formation in respiratory epithelial cells. In the presence of low iron conditions induced by an iron chelator, such as lactoferrin, P. aeruginosa forms an irregular biofilm, with a thin layer of cells. In the absence of lactoferrin, and in the presence of adequate iron concentrations, a normal biofilm is formed. The iron level affects both the formation and maintenance of P. aeruginosa biofilms. A high iron concentration disturbs biofilm formation and stimulates separation of preformed biofilms [21]. Musk et al. reported that iron salts (ferric sulfate, ferric chloride, ferrous sulfate and ammonium ferric citrate), at iron concentrations greater than 100 μM inhibited formation of P. aeruginosa biofilms without effecting growth [21]. The first ten hours of development of initial biofilm formation were unaffected; thus the inhibition is not due to decreased adhesion of cells to the surface. More accurately, excess iron seems to disturb advanced stages of biofilm development, so that few cells stick to the surface by 48 hours [21].
References
1. Dodd M., Prasad S. "Physiotherapy Management of Cystic Fibrosis." Chronic Respiratory Disease 2.3 (2005): 139-49. Web. http://crd.sagepub.com/content/2/3/139.long
2. Høiby, N., B. Frederiksen, and T. Pressler. "Eradication of Early Pseudomonas Aeruginosa Infection." Journal of Cystic Fibrosis 4 (2005): 49-54. Web. http://www.sciencedirect.com/science/article/pii/S1569199305000688
3. Coutinho, H., V. S. Falcão-Silva, and G. Gonçalves. "Pulmonary Bacterial Pathogens in Cystic Fibrosis Patients and Antibiotic Therapy: A Tool for the Health Workers." International Archives of Medicine 1 (2008): 24. Web. http://www.intarchmed.com/content/1/1/24
4. Kahl, B., M. Herrmann, A. Everding, H. Koch, K. Becker, E. Harms, R. Proctor, and G. Peters. "Persistent Infection with Small Colony Variant Strains of Staphylococcus Aureus in Patients with Cystic Fibrosis." Journal of Infectious Diseases 177.4 (1998): 1023-029. Web. http://jid.oxfordjournals.org/content/177/4/1023.long
5. Cystic Fibrosis Foundation Patient Registry 1997. Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation.
6. Jenal U., Malone J. Mechanisms of Cyclic-di-GMP Signaling in Bacteria. Annual Review of Genetics, 40.1 (2006): 385-407. Web. http://www.annualreviews.org/doi/full/10.1146/annurev.genet.40.110405.090423?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed
7. Newell, P. D., S. Yoshioka, K. L. Hvorecny, R. D. Monds, and G. A. O'toole. "Systematic Analysis of Diguanylate Cyclases That Promote Biofilm Formation by Pseudomonas Fluorescens Pf0-1." Journal of Bacteriology 193.18 (2011): 4685-698. Web. http://jb.asm.org/content/193/18/4685.long
8. Merritt, J., D. G. Ha, K. Cowles, W. Lu, D. K. Morales, J. Rabinowitz, Z. Gitai, and G. A. O'Toole. "Specific Control of Pseudomonas Aeruginosa Surface-Associated Behaviors by Two C-di-GMP Diguanylate Cyclases." MBio 1.4 (2010): E00183-10-00183-18. Web. http://mbio.asm.org/content/1/4/e00183-10.full.html
9. "Medical Aspects of Exercise; Benefits and Risks." 1st ed. London: Royal College of Physicians of London, 1991. Print.
10. Orenstein, D. "Cystic Fibrosis and Exercise." Cystic Fibrosis: A Guide for Patient and Family. 4th ed. N.p.: Lippincott Williams & Wilkins, 2011. Print.
11. Webb, A., and M. Dodd. "Exercise and Cystic Fibrosis." Journal of the Royal Society of Medicine 88.25 (1994): 30-36. Web. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1295056/
12. Pérez, M., I.F. Groeneveld, E. Santana-Sosa, C. Fiuza-Luces, L. Gonzalez-Saiz, J. R. Villa-Asensi, L. López-Mojares, M. Rubio, and A. Lucia. "Aerobic Fitness Is Associated with Lower Risk of Hospitalization in Children with Cystic Fibrosis." Pediatric Pulmonology (2013): Web. http://onlinelibrary.wiley.com/doi/10.1002/ppul.22878/abstract;jsessionid=71C57D69F0E5F5010C479391112C3854.f02t02
13. Pianosi, P. "Peak Oxygen Uptake and Mortality in Children with Cystic Fibrosis." Thorax 60.1 (2005): 50-54. Web. http://thorax.bmj.com/content/60/1/50.long
14. Donaldson, S., W. Bennett, K. Zeman, M. Knowles, R. Tarran, and R. Boucher. "Mucus Clearance and Lung Function in Cystic Fibrosis with Hypertonic Saline." New England Journal of Medicine 354.3 (2006): 241-50. Web. http://www.nejm.org/doi/full/10.1056/NEJMoa043891
15. Mall, M., B. Grubb, J. Harkema, W. O'Neal, and R. Boucher. "Increased Airway Epithelial Na Absorption Produces Cystic Fibrosis-like Lung Disease in Mice." Nature Medicine 10.5 (2004): 487-93. Web. http://www.nature.com/nm/journal/v10/n5/abs/nm1028.html
16. Elkins, M., M. Robinson, B. Rose, C. Harbour, C. Moriarty, G. Marks, E. Belousova, W. Xuan, and P. Bye. "A Controlled Trial of Long-Term Inhaled Hypertonic Saline in Patients with Cystic Fibrosis." New England Journal of Medicine 354.3 (2006): 229-40. Web. http://www.nejm.org/doi/full/10.1056/NEJMoa043900
17. Stuart, B., J. Lin, and P. Mogayzel. "Early Eradication of Pseudomonas Aeruginosa in Patients with Cystic Fibrosis." Paediatric Respiratory Reviews 11.3 (2010): 177-84. Web. http://www.sciencedirect.com/science/article/pii/S1526054210000394
18. Hill, D., B. Rose, A. Pajkos, M. Robinson, P. Bye, S. Bell, M. Elkins, B. Thompson, C. Macleod, S. D. Aaron, and C. Harbour. "Antibiotic Susceptibilities of Pseudomonas Aeruginosa Isolates Derived from Patients with Cystic Fibrosis under Aerobic, Anaerobic, and Biofilm Conditions." Journal of Clinical Microbiology 43.10 (2005): 5085-090. Web. http://jcm.asm.org/content/43/10/5085
19. Høiby, N., B. Frederiksen, and T. Pressler. "Eradication of Early Pseudomonas Aeruginosa Infection." Journal of Cystic Fibrosis 4 (2005): 49-54. Web. http://www.sciencedirect.com/science/article/pii/S1569199305000688
20. Fernández-Olmos, A., M. García-Castillo, L. Maiz, A. Lamas, F. Baquero, and R. Cantón. "In Vitro Prevention of Pseudomonas Aeruginosa Early Biofilm Formation with Antibiotics Used in Cystic fibrosis Patients." International Journal of Antimicrobial Agents 40.2 (2012): 173-76. Web. http://www.sciencedirect.com/science/article/pii/S0924857912001641
21. Musk, D., and D. Banko. "Iron Salts Perturb Biofilm Formation and Disrupt Existing Bio- films of Pseudomonas Aeruginosa." Chemical Biology 12 (2005): 789-96. Web. http://www.sciencedirect.com/science/article/pii/S1074552105001389
Edited by Megan Richman, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.
