Triple Symbiotic Relationship between Mealybugs, Tremblaya princeps, and Moranella endobia
Mealybugs
Mealybugs make up a widely distributed group of scaled insects that feed on plant sap and are damaging to many greenhouse and agricultural plants. They are classified under the family Pseudococcidae and order Hemiptera. Mealybugs preferentially inhabit warm and moist niches and can be found worldwide in tropical and temperate climate regions, including indoors on house plants. They are universally recognized as agricultural pests as they feed on plant sap and are vectors of various plant diseases [3][8].
Endosymbionts and “Triple” Symbiosis
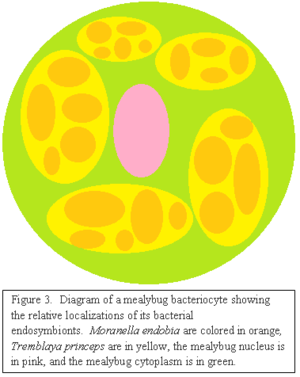
Mealybugs contain specialized oval shaped structures in their abdomen called bacteriomes, which are made up of cells called bacteriocytes. The rod-shaped endosymbionts of mealybugs live within mucoidal spheres that are found in the cytoplasm of these bacteriocytes [5][10]. Previous molecular analysis of 16S rDNA has shown that the insect endosymbionts within these spheres usually belong only to the γ-proteobacteria. However, the endosymbionts in mealybugs were found not only to belong to the γ-proteobacteria, but also the β-proteobacteria. Further analysis has identified the symbiotic mucoidal spheres as the β- proteobacteria themselves, surrounded by a three-membrane bilayer. The γ-proteobacteria were found to be living within these β- proteobacterial spheres and enveloped by only a two membrane bilayer [10]. In other words, mealybugs contain two endosymbionts, the γ-proteobacteria, called Moranella endobia, which reside within the cytoplasm of the β-proteobacteria, identified as Tremblaya princeps [5][1].
Phylogeny
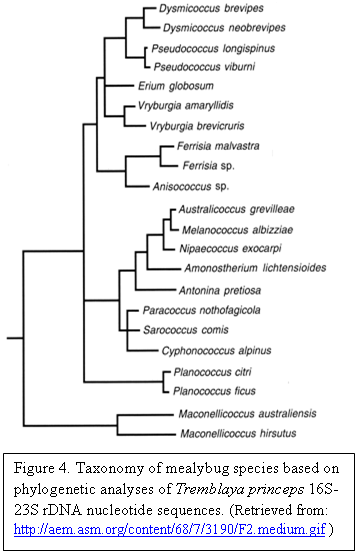
Phylogenetic analysis shows that mealybugs and their β- and γ- endosymbionts evolve through codivergence [4][2]. The primary symbiont, Tremblaya princeps, have been suggested to be vertically transferred from a common ancestor of mealybugs and are considered monophyletic. Tremblaya are considered to be obligate endosymbionts as they are conserved among mealybug species. They are known as the “primary symbiont.” The γ- proteobacteria, Moranella endobia, are believed to have been acquired after Tremblaya was already established. This can be attributed to the observation that Moranella appeared to be absent within basal lineages of mealybugs that still contained Tremblaya. Moranella are referred to as the “secondary symbiont.” Moranella are considered to be polyphyletic as they are thought to have originated separately at different instances in time [4][2][5][7][8].
Genome
Tremblaya princeps, with only 121 genes, has a remarkably small genome. This is attributed to the attrition of unnecessary genes as it is an endosymbiont. For instance, a proposed rationalization for Tremblaya’s unusually small genome is due to its particularly unique endosymbiotic relationship with Moranella. Moranella is suggested to have taken over some of Tremblaya’s responsibilities in nutrient production. This is thought to have allowed Tremblaya to efficiently stop expressing genes that were complementary with the genome of Moranella. This is supported by the observation that Tremblaya pseudogenes have functional counterparts in the Moranella genome. The genome of Moranella endobia, with 538, 294 base pairs, is approximately four times the size of Tremblaya princeps’ [6].
Trophic Dependencies
Mealybugs depend on a unique endosymbiotic relationship in which they have a primary symbiont, Tremblaya princeps, which also contain an endosymbiont, Moranella endobia. The endosymbionts are shown to be not only beneficial to the mealybug but essential to their survival as a study where both endosymbionts were impaired was shortly followed by the death of the mealybugs [5]. Mealybugs feed exclusively on nutrient deficient phloem sap which has low levels of proteins and lipids, but is abundant in carbohydrates. The mealybug is able to derive the required amount of lipids from the carbohydrates present. However, there still an insufficient amount of essential amino acids in the sap [5]. Due to the mealybug’s inability to convert plant sap into all of the required essential nutrients, it depends on the metabolic products of its endosymbionts to supplement its diet. Tremblaya princeps has genes involved in the synthesis of the essential amino acids; however, none of the pathways are complete. Moranella endobia supplements the remaining complementary genes needed to complete the essential amino acid pathways in Tremblaya. Therefore, Tremblaya are responsible for certain steps in the conversion of the sap into amino acids and the remaining steps are handled by Moranella [6]. The nested nature of these endosymbionts is thought to allow for the efficient exchange of metabolites between the two. The endosymbionts are said to be involved in a complementary symbiosis as neither bacterium, on their own, contains the complete biomachinery required to convert the plant sap into the necessary essential amino acids. Therefore, a combination of gene products from both endosymbionts is considered to be necessary for the complete synthesis of essential amino acids, such as threonine and tryptophan. Furthermore, the complete synthesis of amino acids such as isoleucine, phenylalanine and arginine are suggested to require gene products from the mealybug in addition to the combination of gene products required from both of its endosymbionts [6].
Significance and Further Research
The prokaryote-within-a-prokaryote symbiotic nature of mealybugs is one of the first observed in nature. The unique nature of the interaction between the β- endosymbiont, the γ-endosymbiont, and the host insect is of interest for further research as it has been hypothesized to give further insight to the evolutionary origin of mitochondria, chloroplasts, and other organelles of eukaryotes. The endosymbiotic theory for the origin of mitochondria and chloroplasts proposes that these organelles descended from prokaryotic bacteria that had survived endocytosis by another cell, becoming endosymbionts. Over time, the endosymbionts became increasingly dependent on host mechanisms as their genes were transferred to the host cell chromosome, continuingly reducing their genomes. Mitochondria originated from proteobacteria and chloroplasts originated from cyanobacteria. The β-endosymbionts in mealybugs are similarly believed to have arisen from endocytosis. Thus, further investigation could lead to a better understanding of the evolutionary origins of eukaryotic cells [5][9].
References
(1) Baumann, L. and Baumann, P. "Cospeciation between the primary endosymbionts of mealybugs and their hosts." Current Microbiology, 2005, DOI: 10.1007/s00284-004-4437-x
(2) Baumann, L., Thao, M.L., Hess, J.M., Johnson, M.W. and Baumann, P. "The genetic properties of the primary endosymbionts of mealybugs differ from those of other endosymbionts of plant sap-sucking insects.” Applied and Environmental Microbiology, 2002, DOI: 10.1128/AEM.68.7.3198–3205.2002
(3) Beardsley, J.W. “Mealybugs of California with Taxonomy, Biology, and Control of North American Species (Homoptera: Coccidea; Pseudococcidae).” The Quarterly Review of Biology, 1969; 44 (1): 89-91.
(4) Downie, D.A. and Gullan, P.J. “Phylogenetic congruence of mealybugs and their primary endosymbionts.” Evolutionary Biology, 2005, DOI: 10.1111/j.1420-9101.2004.00834.x
(5) Kono, M., Koga, R., Shimada, M. and Fukatsu, T. “Infection dynamics of coexisting beta- and gammaproteobacteria in the nested endosymbiotic system of mealybugs.” Applied and Environmental Microbiology, 2008, DOI: 10.1128/AEM.00250-08
(6) McCutcheon, J.P. and von Dohlen, C.D. “An interdependent metabolic patchwork in the nested symbiosis of mealybugs.” Current Biology, 2011, DOI: 10.1016/j.cub.2011.06.051
(7) Munson, M.A., Baumann, P. and Moran, N.A. “Phylogenetic relationships of the endosymbionts of mealybugs (Homoptera: Pseudococcidae) based on 16S rDNA sequences.” Molecular Phylogenetics and Evolution, 1992, DOI: 10.1016/1055-7903(92)90032-C
(8) Thao, M.L., Gullan, P.J. and Baumann, P. “Secondary (γ-proteobacteria) endosymbionts infect the primary (β-proteobacteria) endosymbionts of mealybugs multiple times and coevolve with their hosts.” Applied and Environmental Microbiology, 2002, DOI: 10.1128/AEM.68.7.3190–3197.2002
(9) Timmis, J.N., Ayliffe, M.A., Huang, C.Y. and Martin W. “Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes.” Nature Reviews Genetics, 2004, DOI: 10.1038/nrg1271
(10) von Dohlen, C.D., Kohler, S., Alsop, S.T. and McManus, W.R. “Mealybug beta-proteobacterial endosymbionts contain gamma-proteobacterial symbionts.” Nature, 2001, DOI: 10.1038/35086563