Trypanosome Life Cycle
Introduction
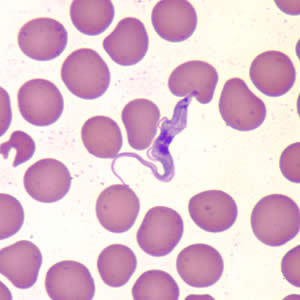
Trypanosoma brucei and its related subspecies are parasitic hemoflagellate protists that are implicated in a severe meningoencephalitic disease, African trypanosomiasis, more commonly known as “sleeping sickness.” Sleeping sickness itself is identified as a neurological disorder preceded by an acute lymphatic infection. The disease garners its name from the effect it has of creating severe fatigue in a patient followed by bouts of mania and eventually causing coma and death. It is known to have affected up to 36 sub-Saharan countries with an estimated 300,000 new infections each year. A more complete understanding of the life cycle of this particularly fatal disease has been incited by organizations such as the WHO in order to create novel treatments for the disease before it develops into a much more deadly issue.
Protists of the genus trypanosoma are a fairly typical eukaryotic flagellates with fully intact and functional organelles including mitochondria and nuclei (which is atypical of most obligate parasites). T. brucei mitochondria contain kinetoplasts, condensed regions of mitochondrial DNA maintained within the mitochondria. The kinetoplast is closely associated with the flagellum of the trypanosome and is believed to be an essential part of the regulation of flagellar motility as the parasite enters different stages of its life cycles. Perhaps the most fascinating and unique characteristic of T. brucei is the ability to alter the composition of a dense glycoprotein coat in order to evade the specific immune response mechanisms of the host body.
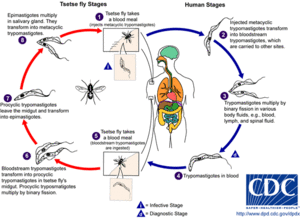
Ecologically, T. brucei is an obligate parasite and requires the presence of a host cell to function. However, it is not readily transmittable between mammalian hosts and facilitates the use of an insect vector: the flies of the genus Glossina, otherwise known as the Tsetse flies. These flies are endemic to 36 sub-Saharan countries and are found, unsurprisingly, within the range of trypanosomiasis infections. Tsetse flies themselves are not fatally infected by the parasites as their hemolymph is not capable of providing the amount of sustenance necessary for trypanosomal reproduction, but they are mildly affected by the trypanosomes presence.
Within both the human and fly hosts, T. brucei undergoes a series of wildly pleomorphic events that are necessary for the successful transmission of the parasite. For example, within the fly the parasite expresses a coat that is made of several different procyclin molecules. Before transmission to the human host, the trypanosome gathers the previously mentioned variable surface glycoprotein (VSG) coat. In fact, for almost all trypanosomes, infectivity from the insect vector to the mammal host correlates with the expression of a dense VSG coat.
Once within the host, T. brucei undergoes several morphological and enzymatic changes to facilitate inter-host transfer of the parasite to various organ systems including the lymphatic system and the central nervous system. Each of these transfers are facilitated in different ways and the timing and the severity of the infection vary depending on which subspecies of T. brucei are causing the infection. Subspecies rhodesiense is a form of the disease categorized by an acute onset of neurological complications and requires treatment within days of infection before the disease becomes fatal. Subspecies gambiense is a much more chronic form of the disease with cyclical infective patterns marked by a rapid change in the VSG coat of the parasite. While there is not a complete understanding of the methods by which each of these subspecies function, researchers have been collecting a vast amount of information regarding the developmental cycles of T. brucei in order to effectively combat the disease.
Antigenic Variation
Trypanosomes in general exist in a very exposed and vulnerable ecological niche within their human hosts: the bloodstream. In this environment most pathogens would be susceptible to not only the damaging effects of the innate immune response (inflammation, leukocytes, etc) but also the adaptive immune response (antibodies, killer T cells, etc.). Some hemolytic bacteria and protists have developed methods to avoid the innate immune response through the expression of a lipo-polysaccharide coat (gram-negative bacteria only) or even thick cellular walls, but it is difficult for either of these methods to avoid the specific targeting mechanisms of the adaptive immune system.
Trypanosomes have evolved to thwart the adaptive immune response of the host by maintaining a variant surface glycoprotein (VSG) coat which expresses one of ~1500 surface glycoprotein genes. A new set of surface glycoproteins are expressed in T. brucei outer membranes in approximately 1 out of every 100 cell divisions. This ensures that once the host mounts an effective complementary immune response to the predominantly expressed VSG variant, the infection will persist into the next generation until the host mounts another complementary response. This variable expressive pattern is the main reason patients with chronic trypanosomiasis show cyclical bouts of infection followed by a period of relative latency. By the time the host’s immune system identifies and synthesizes antibodies to combat certain trypanosomes, a percentage of the total trypanosome population has evaded detection and continued the infection.
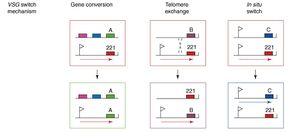
For each trypanosome organism, only one VSG gene is expressed at a time. Genomic research has identified ~20 VSG expression sites (ES) along a telomere where VSG mRNA is transcribed. Each ES is a polycistronic unit where 13 different genes are expressed including the VSG gene. Further analysis of the trypanosome genome has shown that there are upwards of 1500 “silent” VSG genes that are not associated with the polycistronic ES. 1250 of these silent genes are hidden on subtelomeres with several silent genes located on the same chromosome. ~150 of these silent genes are found in the telomeres of tightly packed 80-100kb minichromosomes. These minichromosomes contain only one actual coding gene on an inactive polycistronic ES unit while most of the minichromosome consists of non-coding palindrome sequences.
The particular mechanisms of VSG gene switching can vary depending on the source of the VSG gene itself. VSG genes associated with a VSG ES, but are not expressed may simply be preferentially activated by the cell during cell replication. For a silent VSG gene found on a minichromosome, the typical method of activation is to actually transfer an entire coding sequence from an inactive telomere to a telomere containing a promoter sequence replacing the VSG gene that was previously located there. Silent genes found on the subtelomeres also transfer through this method, but these complexes transfer only one particular VSG gene rather than an entire ES from one telomere to another.
These advantageous VSG coats are only found within the metacyclic stages of the trypanosomes life cycle which exist only in the salivary gland of the Tsetse fly and in the mammalian host bloodstream. Within the midgut of the tsetse fly, a separate series of cell surface molecules known as procyclins dominate the cell membrane.
Development within Insect Vector
Human African trypanosome infections follow a cyclical path between human hosts and an insect vector. Within this cycle the insect vector is inoculated with a particular form of the trypanosome from the human host which then transforms into a form that the fly can deliver to the human host. Throughout this cycle the trypanosome enter developmental stages that either undergo cell division or prepare themselves for delivery into the complementary host. The current understanding of the trypanosome development within insect host is that the trypanosome will enter three major stages of development: the procyclic form, the epimastigote and the metacyclic form. Of these three forms, only the procyclic and the epimastigote will multiply and the metacyclic form is only adapted to be transferred to the human host.
As the fly takes in a blood meal from a human host, there are two major forms of T. brucei: the slender (normal) forms and the stumpy (non-replicating) forms. Only the stumpy forms of the trypanosome are thought able to survive the onslaught of potent proteases present in the saliva and midgut of the tsetse fly. These stumpy forms transform into their next stage through a sequence of reactions that are not well understood. Some researchers propose that the proteases present in the midgut of the fly could provide some unknown, “natural trigger.” Others propose that in addition to the protease function there are a series of reactions within the trypanosome that are triggered by the cold shock the trypanosome would experience in travelling between an endotherm and an ectotherm. This cold shock theory rests on the idea that these low temperature shocks expose the parasite to higher levels of citrate of cis-aconitate or even inhibit the effect of tyrosine phosphatase (an enzyme associated with various cellular differentiation mechanisms).
Irregardless the mechanism, the stumpy forms quickly transform into the procyclic stage where the trypanosomes attach to the cells of the ectoperitrophic space and begin to transform into a more motile form that travels along the proventriculus of the fly midgut, known as a late procyclic form or a trypomastigote (mastigo- v. whip in greek. Referring to the whipping motion of the flagella). This trypomastigote travels quickly through the proventriculus of the fly until it reaches the fly’s salivary gland. The kinetoplast (originally attached to the flagella) is transferred within the cell which causes the trypomastigote change into the long epimastigote form. The long epimastigote settles on the basolateral portion of the salivary gland membrane and asymmetrically divides forming the short epimastigote stage. Short epimastigotes are currently considered to be the form of the trypanosome that colonizes the salivary gland of the Glossina. This developmental stage is currently considered to be responsible for the production of metacyclic trypanosomes prior to infection of the mammalian host cell. Very little is actually known about the cell biology of each stage differentiation. The transfer of the kinetoplast and its overall function are also not well understood, but it is known that relocating the kinetoplast is essential to the production of long epimastigotes.
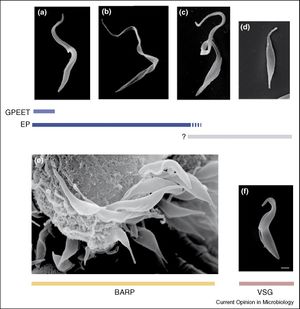
Throughout each of these developmental stages, the trypanosome cellular glycoprotein coat is modified in a vaguely ordered fashion. Even from as early a stage in the fly infection as the transfer of the stumpy infective form, the VSG coat that surrounds the trypanosome is shed off completely and is replaced with a midgut protease resistant coat of a particular group of proteins known as procyclins. Procyclins themselves are chained surface glycoproteins with repeating segments of either glu-pro (EP) or gly-pro-glu-glu-thr (GPEET). Both of these types of procyclins have varying numbers of isoforms that are expressed preferentially by different developmental stages. EP-procyclins are expressed as three distinct isoforms: EP1, 2, and 3.
With regards to the time it takes for the different procyclic stage glycoprotein coats to arise in the cell membrane, typically a full coat will have grown in ~4 hours. For procyclic stage trypanosomes that had recently just infected a tsetse fly, the predominant version of procyclin that is found is GPEET-procyclin. Procyclic trypanosomes with large numbers of GPEET-procyclin and low levels of EP-procyclins are marked as “early stage Procyclic”. GPEET-procyclin coats typically last until about the 4th or 5th day of infection before higher concentrations of EP-procyclin molecules are found within them. During the late stage procyclic stage almost no GPEET-procyclins are found and EP1 and EP3-procyclins predominate the cellular glycoprotein coat. EP-2 procyclins are found in large numbers throughout every procyclic stage.
The long epimastigote stage glycoprotein coat structure is not as well understood as that of the procyclic stage and there are many inconsistencies with the data that has been presented. Certain protease-resistant early epimastigote coat proteins have been identified in Trypanosoma Congolese (Non-human Mammalian Trypanosomiasis), but these protease-resistant surface (PRS) molecules have not been found in any other form of Trypanosome and are assumed to be a species specific variant. In T. brucei short epimastigotes, cell surface proteins consist mainly of an alanine-rich protein (BARP). BARPs in T. brucei are expressed from several sets of large multigene complexes and have several different isomers. It is possible that these multiple BARP forms are used by the epimastigote to promote adhesion to the salivary gland cells, but it is not currently known.
As the fly portion of the trypanosome life cycle comes to a close, the short epimastigotes begin to produce large numbers of metacyclic trypanosomes in the salivary glands of Glossina. In the salivary gland, the newly-spawned metacyclic trypanosomes will reacquire the VSG coat and completely shed any expression of a procyclin or BARP cell surface protein. The metacyclic trypanosome requires the reacquisition of the VSG coat to survive the immune response of the human host. It is interesting to note that although the VSG coat is essential for the metacyclic infective form to survive in the human host, the procyclin coat proteins (GPEET and EP) are non-essential for the proliferation and transfer of trypanosomes within the Glossina midgut.
Development and Pathogenicity within a Human Host
Once a metacyclic Trypanosoma brucei enters the mammalian bloodstream, a regulated cascade of biochemical signals transforms the metacyclic trypanosome into the typical slender form of a trypanosome. This form flagellar, bloodborne pathogen alone causes the devastating effects of African trypanosomiasis and because of this, an incredible amount of research has developed to fully understand not only the disease itself but how the trypanosome itself traverses the various and complex labyrinth of the human body. As stated earlier, there must be a distinction in the type of African trypanosomiasis as the rhodosiense subspecies and the gambiense subspecies cause different symptoms altogether. Recall that rhodosiense infection is far more acute and severe and can quickly lead to death while gambiense is a more chronic and more actively employing the use of antigenic variation.
Upon the site of metacyclic trypanosome injection, one of the first sign of trypanosome infection that occurs in the rhodosiense subspecies is the presence of deep lesions as the trypanosome tears its way through the capillary beds of the human skin and proceeds to infect larger and more favorable blood vessels. Gambiense infections do not cause the lesions of the rapidly spreading rhodosiense, but it indeed spreads throughout the capillary bed in a similar fashion and leads to the same large vasculature. These symptoms will persist for a few days and then, while the lymph node is draining from the infection, the trypanosome will pass through into the lymph node and begin infection of the lymphatic system. This infection causes severe bouts of headaches, fever, and an increased level of fatigue in the patients. The trypanosome itself is digesting the cells of the lymphatic system systematically and causing the body to respond harshly. In the case of the rhodosiensis, the effects of such an acute attack could cause the body to overrespond in its attempts to rescue the body and possibly lead to edema, pancarditis and congestive heart failure. Fortunately, this form of trypanosomiasis is easily identified and diagnosable. If the disease is caught early within the hemolymphatic stage of the infection the risk involved with treatment is dramatically lowered. However, gambiensis does not show these severe symptoms of its brasher cousin, but it is far more insidious. It leads to a series of general immune responses and then, causes a swelling of the lymph nodes, which are both easily mistaken for other diseases. One unique symptom of gambiense infection is the presence of a swollen section of the lymph nodes of the trapezius region of the upper back that is known as “Winterbottom’s sign”.
The most deadly and serious symptoms of trypanosomiasis comes from when the trypanosomes invade the organ systems themselves. After a few weeks with rhodensiensis and after as much as a couple of years with gambiensis, evidence of increased immune response in tissues other than the blood begins to appear, signaling their infection. Trypanosomes have perhaps the most devastating effect on the human host by even attacking the central nervous system (CNS). Recent studies have shown that trypanosomes invade the central nervous system directly through the blood brain barrier (BBB) as well as through portions of the circumventricular organs. Apparently, the trypanosomes somehow render themselves permissible through the BBB by expressing a parasite version of cysteine protease which increases the number of changes in the oscillatory patter of Calcium ions in the cell.
As the meningoencephalitic stage of the disease progresses, the patient will show a drastic change in their sleeping habits, where they sleep all day during the day and have intermittent periods of insomnia at night. Eventually in the most devastating periods of the disease, personality changes in the form of anger and depression can onset followed soon after by incapacitation of basic reasoning or mental processing. Severe ataxic dyskinesia eventually sets in and the patient can lose the ability to coordinate their actions at all.
Beyond their pathogenic life cycles, the development of the “stumpy” trypanosomes are a potentially crucial part of the infective pathway of T. brucei. The stumpy trypanosomes develop from a quorum sensing apparatus that promotes several organelle rearrangements as well as an arrest of several mitotic gateways. The particular protein involved in the quorum sensing is an unknown theoretical molecule simply referred to as “stumpy inducing factor”. As the stumpy forms develop, they lose the ability to reproduce entirely and instead lie near the surface capillary beds to infect the blood meal of a potential insect vector.
For a large period of time, it was assumed that differentiation of the slender form to the stumpy form was essential to the life cycle of T. brucei. However, recently a study has shown that both slender and stumpy forms of the trypanosome have the capacity to complete an entire life cycle throughout an insect vector, back into a mammalian host.
References
1. Krafsur, K. "Tsetse flies: Genetics, evolution, and role as vectors" Infection, Genetics and Evolution 2009. Volume 9 p.124-141
2. Taylor, J. Rudenko, G. "Switching trypanosome coats: what's in the wardrobe?" Trends in Genetics. 2006. Volume 22 No. 11
p.614-621
3. Donelson, J. "Antigenic variation and the African trypanosome genome" Acta Tropica. 2003. Volume 85 p.391-404
4. Nikolskala, O. Lima, A. Kim, Y. Lonsdale-Eccles, J. Fukuma, T. Scharfstein, J. Grab, D. "Blood-brain barrier traversal by
African trypanosomes requires calcium signaling induced by parasite cysteine protease" The Journal of Clinical Investigation.
2006. Volume 116 No. 10 p.2739-2747
5. Fenn, K. Matthews, K.R. The cell biology of Trypanosoma brucei differentiation. 2007.
Current Opinion in Microbiology Volume 10 p.539-546
6. Vassella, E. Oberle, M. Urwyler,S. Renggli, C.K. Studer, E. Hemphill, A. Fragoso, C. Butikofer, P. Brun,R. Roditi,I.
Major Surface Glycoproteins of Insect Forms of Trypanosoma brucei Are Not Essential for Cyclical Transmission by Tsetse 2009.
PLoS Volume 4 Issue 2 e4493.
7. Pays, E. Regulation of antigen gene expression in Trypanosoma brucei. 2005. Trends in Parasitology. Volume 21
Number 11. 517-520
8. Kennedy, P.G.E. Human African trypanosomiasis - neurological aspects 2006. J Neurol Volume 253 p.411-416
9. Roditi, I. Lehane, M.J. Interactions between trypanosomes and tsetse flies. 2008. Current Opinion in Microbiology Volume 11.
p. 345-351
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
