Type Six Secretion System (T6SS)
Introduction/Function
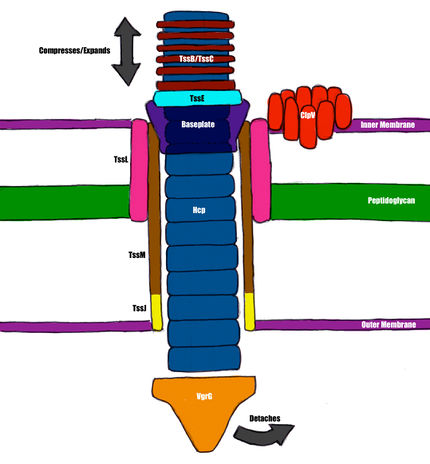
The Type VI Secretion System is the most recently discovered [7] of the six mechanisms for effector secretion elucidated in gram-negative bacteria, particularly notable for its significant structural homology with T4 bacteriophage [18]. The basic structure consists of thirteen proteins necessary for function [18]. The compounds delivered by the T6SS have not been characterized in most bacterial species. Three substrates have been identified in Pseudomonas aeruginosa: two have peptidoglycan hydrolysis activity, and are secreted into the recipient periplasm [15].
Structure
Hcp
Hcp, or Hemolysin Coregulated Protein, is homologous to the major tail protein, gpV, in phage [14]. It exists as hexamers that have been induced to form nanotubes in vitro, but this process has yet to be demonstrated in vivo [3]. It is theorized to form a tail of defined length, with an external diameter of 9 nm and an internal diameter of 4 nm [18]. Tubule formation is likely a result of VgrG recruitment, a process similar to the mechanism that occurs in phage, but the association between Hcp and VgrG has yet to be detected experimentally [18]. The internal diameter of the tubule suggests that the T6SS can theoretically transport globular proteins up to 50 kDa [18].
VgrG
VgrG, or Valine-Glycine Repeat Protein G, is homologous to the gp27/gp5 complex, or the tailspike of bacteriophage T4 [14]. The C-terminal tip residues have been theorized to have fibronectin and peptidoglycan-binding properties, as well as general adhesion and actin modification activity in silico, but most of these properties have yet to be tested experimentally [18]. The tip of VgrG is too narrow to allow the passage of protein substrates, and therefore VgrG probably dissociates upon entry to the recipient cell, exposing the hollow Hcp tubule [18].
TssB/TssC
These two proteins complex in a manner homologous to the T4 sheath [7]. They are rendered contractile by their two-conformation nature [4] and likely provide the mechanical force for membrane perforation [18]. Cross-sections of these complexes have been experimentally determined to be hollow, with a 10nm diameter, which is large enough to house the Hcp tubule [7]. There is evidence to suggest that the disassembly of this complex is regulated by ClpV, an intracellular signaling molecule [5].
TssE
This protein shows significant structural homology with gp25; the most significant structural component of the bacteriophage baseplate [4]. In bacteria, it is necessary for the assembly of the T6SS [4], and is theorized to be involved in the association of the secretion system with the internal membrane [18].
TagL/TssL
TagL is a protein, required for T6S activity, that contains PG-binding motifs and likely acts as a cell wall binding agent [2]. It associates with TssL, a hook-shaped protein that is localized mainly to the cytoplasm and has only one inner membrane-spanning sequence [8].
TssJ
TssJ is a lipoprotein in the outer membrane, associated with an acylated N-terminal cysteine [1]. It possesses a sequence, with low conservation, that binds TssM, and this low conservation may be an indication that the sequence aids in specificity/determination of T6SS activity [9].
TssM
This envelope-spanning protein has three transmembrane sequences, and interacts with TagL on the cytoplasmic side, and TssJ on the C-terminal, periplasmic side [18]. It is thus critical for the assembly of the membrane-bound components, which have been theorized to form a ring around the phage-like components, similar to structures described in Type III and IV Secretion Systems [10][19]. One section of TssM that is exposed to the cytoplasm contains a Walker motif (a sequence that is traditionally associated with ATP binding and hydrolysis) [18]. The functionality of this sequence is unclear, as it is unnecessary for assembly in Edwardsiella tarda [20], but absolutely necessary, and under ATP binding/hydrolysis control, in Agrobacterium tumefasciens [13].
Other Components (TssA, TssF, TssF, TssK)
These proteins have a high degree of conservation across type six secretion systems, and have been experimentally determined to be required for T6SS function, but their exact purpose remains unclear [18].
Ecophysiological Context
Quorum Sensing
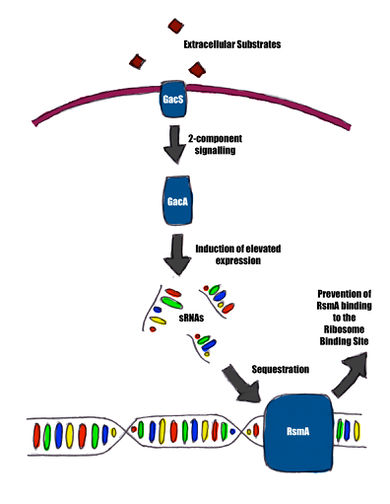
Most quorum sensing research with regard to the T6SS has been done on Vibrio cholerae, with focus on its pathogenesis [11]. In Pseudomonas aeruginosa, it has been shown that LasR, a quorum regulator, represses the T6SS [12]. A particularly important system to bacterial interactions in the context of the T6SS is the Gac/Rsm pathway: a system that regulates roughly 500 genes, including the T6SS in Pseudomonas aeruginosa, Pseudomonas fluorescens and Pseudomonas syringae [18]. Extracellular substrates generated by other bacteria signal a two-component system composed of GacS and GacA, which signals for the expression of several small regulatory RNAs (sRNA) [18]. These small RNAs sequester RsmA/CsrA, a translation inhibitor that controls a variety of genes, which include genes that code for the T6SS [18].
Surface Recognition and Biofilms
There is a large change in gene expression between planktonic bacteria and those operating as a biofilm. While the specific regulator of expression has not yet been found, Pseudomonas aeruginosa biofilms can contain high levels of T6SS components [16], and surface growth of the organism has been shown to activate a signaling pathway that post-translationally activates the HSI-I (Hcp Secretion Island-I, a group of genes of related function to the hemolysin coregulated protein that share the same regulatory factors) through a variety of intermediates [17].
A Specific Example: Ferric Uptake Regulator Protein
The ferric uptake regulator protein, Fur, is principally involved with the regulation of cellular iron uptake and maintenance processes, though it is involved in the regulation of a wide array of seemingly unrelated genes [18]. Fur generally acts as a repressor, and it binds a specific sequence called the Fur box within promoters, after stimulation with Fe(II) [18]. Fur has been shown to repress T6SS production in Edwardsiella tarda, a fish pathogen, and loss of the T6SS renders E. tarda less virulent by two orders of magnitude [18]. In Enteroagreggative Escherichia coli, a diarrhea-causing bacterium that forms biofilms on the intestinal mucosa, the expression of the relevant T6SS has been shown to be controlled by Fe(II) via the Fur pathway, among other control mechanisms [6].
References
[1] Aschtgen M.-S., C. S. Bernard, S. De Bentzmann, R. Lloubès, and E. Cascales. 2008. SciN is an outer membrane lipoprotein required for type VI secretion in enteroaggregative Escherichia coli. J. Bacteriol. 190:7523–7531.
[2] Aschtgen M.-S., M. Gavioli, A. Dessen, R. Lloubès, and E. Cascales. 2010. The SciZ protein anchors the enteroaggregative Escherichia coli Type VI secretion system to the cell wall. Mol. Microbiol. 75:886–899.
[3] Ballister E. R., A. H. Lai, R. N. Zuckermann, Y. Cheng, and J. D. Mougous. 2008. In vitro self-assembly of tailorable nanotubes from a simple protein building block. Proc. Natl. Acad. Sci. U.S.A. 105:3733–3738.
[4] Basler M., M. Pilhofer, G. P. Henderson, G. J. Jensen, and J. J. Mekalanos. 2012. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483:182–186.
[5] Bönemann G., A. Pietrosiuk, A. Diemand, H. Zentgraf, and A. Mogk. 2009. Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion. EMBO J. 28:315–325.
[6] Brunet Y. R., C. S. Bernard, M. Gavioli, R. Lloubès, and E. Cascales. 2011. An epigenetic switch involving overlapping fur and DNA methylation optimizes expression of a type VI secretion gene cluster. PLoS Genet. 7:e1002205–e1002205.
[7] Cascales E. E., and C. C. Cambillau. 2012. Structural biology of type VI secretion systems. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 367:1102–1111.
[8] Durand E., A. Zoued, S. Spinelli, P. J. H. Watson, M.-S. Aschtgen, L. Journet, C. Cambillau, and E. Cascales. 2012. Structural characterization and oligomerization of the TssL protein, a component shared by bacterial type VI and type IVb secretion systems. J. Biol. Chem. 287:14157–14168.
[9] Felisberto-Rodrigues C. C., E. E. Durand, M.-S. M. Aschtgen, S. S. Blangy, M. M. Ortiz-Lombardia, B. B. Douzi, C. C. Cambillau, and E. E. Cascales. 2011. Towards a structural comprehension of bacterial type VI secretion systems: characterization of the TssJ-TssM complex of an Escherichia coli pathovar. PLoS Pathog. 7:e1002386–e1002386.
[10] Fronzes R., P. J. Christie, and G. Waksman. 2009. The structural biology of type IV secretion systems. Nat. Rev. Microbiol. 7:703–714.
[11] Jani A. J., and P. A. Cotter. 2010. Type VI Secretion: Not Just for Pathogenesis Anymore. Cell Host Microbe 8:2–6.
[12] Lesic B., M. Starkey, J. He, R. Hazan, and L. G. Rahme. 2009. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Annu. Rev. Microbiol. 155:2845–2855.
[13] Ma L.-S., J.-S. Lin, and E.-M. Lai. 2009. An IcmF family protein, ImpLM, is an integral inner membrane protein interacting with ImpKL, and its walker a motif is required for type VI secretion system-mediated Hcp secretion in Agrobacterium tumefaciens. J. Bacteriol. 191:4316–4329.
[14] Pell L. G., V. Kanelis, L. W. Donaldson, P. L. Howell, and A. R. Davidson. 2009. The phage lambda major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc. Natl. Acad. Sci. U.S.A. 106:4160–4165.
[15] Russell A. B., R. D. Hood, N. K. Bui, M. LeRoux, W. Vollmer, and J. D. Mougous. 2011. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343–347.
[16] Sauer K., A. K. Camper, G. D. Ehrlich, J. W. Costerton, and D. G. Davies. 2002. Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184:1140–1154.
[17] Silverman J. M., L. S. Austin, F. Hsu, K. G. Hicks, R. D. Hood, and J. D. Mougous. 2011. Separate inputs modulate phosphorylation-dependent and -independent type VI secretion activation. Mol. Microbiol. 82:1277–1290.
[18] Silverman J. M., Y. R. Brunet, E. Cascales, and J. D. Mougous. 2012. Structure and Regulation of the Type VI Secretion System. Annu. Rev. Microbiol. 66:120702150313000.
[19] Worrall L. J., E. Lameignere, and N. C. J. Strynadka. 2011. Structural overview of the bacterial injectisome. Curr. Opin. Microbiol. 14:3–8.
[20] Zheng J., and K. Y. Leung. 2007. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 66:1192–1206.