User:Cortez10
Classification

Ustilaginoidea virens
U. virens (anamorphic form)
Subclass: Incertae sedis
Order: Incertae sedi
Family: Incertae sedis
Genus: Ustilaginoidea
Villosiclava virens (V. virens)
V. virens (teleomorph form)
Subclass: Sordariomycetes
Order: Hypocreales
Family: Clavicipitaceae
Genus: Villosiclava
Use NCBI link to find]
Species
|
NCBI: [1] |
Genus species": Ustilaginoidea and Villosiclava
Background and Significance
Rice false smut (RFS) has become a considerable threat to global rice production, exerting a notable impact to affect both yield and grain quality. The fungus Villosiclava virens (V. virens), along with its anamorphic form Ustilaginoidea virens (U. virens), belongs to the Kingdom Fungi, specifically within Phylum Ascomycota and Class Ascomycetes. U. virens, the anamorph form responsible for sexual reproduction, is classified further into Subclass Incertae sedis, Order Incertae sedi, Family Incertae sedis, and Genus Ustilaginoidea. The fungal life cycle involves both asexual and sexual reproduction. The asexual stage (anamorphs) promotes rapid propagation and the sexual stage (teleomorphs) leading to efficient propagation and genetic recombination to generate new strains. The teleomorph form, V. virens, is found in Subclass Sordariomycetes, Order Hypocreales, Family Clavicipitaceae, and Genus Villosiclava, adding layers of complexity to the fungal life cycle (Fan et al., 2016). The flexibility of the fungus to adapt to changing environmental conditions further demonstrates the status of the fungus to cause the disease, rice false smut, to be considered a significant rice pathogen in agriculture production. Rice false smut has a notable impact on rice cultivation, particularly in the regions of China, the United States, and India. (Fan et al., 2016). China is known to tackle an expansive rice false smut disease incidence of 3.06 million hectares of rice fields, which results in an annual yield loss of 158.6 million kilograms. The Yangtze River basin remains a hot spot for rice false smut despite immense efforts between 2008 and 2016 despite attempts to reduce the disease across 6.9 hectares. In these regions, rice false smut incidence in the region continues to persist, ranging from 19% to 40%. India, faces similar struggles of incidence rates in rile tillers that fluctuate between 5% and 85%. These rice tillers are pivotal for grain production because they are susceptible to rice false smut and lead to rice yield loss ranging from 0.2% to 49% (Liu et al., 2023).
Genome
The genome of the Villosiclava virens (V. virens) genome has significantly enabled our understanding of the fungus pathogenicity with rice plants. The genome UV-8b and IPU010 both have around 39.4 megabases and 33.6 megabases, which have provided insight into the evolutionary association of V. virens. The fungus V. virens secrete proteins that are released in the host’s extracellular environment and the fungus synthesizes their natural product known as secondary metabolites. However, these secondary metabolites are not essential for growth and reproduction.V. virens also have many effector proteins, such as UV_1261, UV_2508, and UV_2286 that play a pivotal role in the suppression of host defense of rice host plants, which enables them to promote infection. The virulence of the pathogen factors, including UvSUN2, UvPRO1, and Uvt3277 also enable the fungus to evade the host defense while it acquires nutrients from the rice host plant (Sun et al., 2020).
Infection
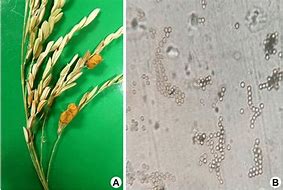
Rise false smut (RFS) infections begin when the fungus Ustilaginoidea virens (U.virens) attacks and infiltrates the rice floral organs. The fungus produces a network of white hyphae for the infection process to occur in the floral organs. As the infection progresses, the fungus then manifests a pigmented dark green chlamydospore within the rice spikelets. In late autumn, the temperature fluctuates throughout the night and day, and the chlamydospore balls are covered with sclerotia. The sclerotia offer the chlamydospore some defense to be resilient The rice false smut also causes blemishes on the rice plant and causes the contamination of the straw and rice grain (Zhang et al., 2014).
Production of Mycotoxins
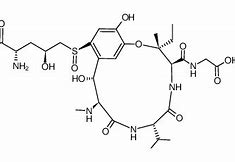
The fungus releases mycotoxins such as ustiloxins and ustilaginoidins. Villosiclava virens (V. virens) can also produce mycotoxins, ustiloxins, and ustilaginoidins. Ustiloxins are water soluble and there are six identified structures, including A-D. and F. A consortium of genes is proposed to have control over the regulation of the ribosomal synthesis of ustiloxins (Wang et al., 2019). There is also the implication that ustiloxins and ustilaginoidins have pathways and the genes involved are not certain (Zhang et al., 2014). It is known that Ustiloxins A and B are primarily abundant in rice false smut balls. In the early stage of maturation, Ustiloxins A and B can be detected in the middle layer of the mycelia and are also found in immature chlamydospores. While ustilaginoidea can be found in rice's false smut balls or within layers of chlamydospores (Wang et al., 2019). Due to the possible resilience of the fungus U. virens, the production of toxins such as ustiloxin can be affected due to environmental conditions. Ustilotoxins and cyclopeptide mycotoxins have been shown to be harmful to humans and animals, but there has been little research on toxicology studies to reveal the significant health effects affiliated with mycotoxins produced U. virens. For instance, zebrafish exposed to a dose-dependent amount of ustilotoxin A experienced decreased hatching rates, higher mortality rates, and impairment of motor function in zebrafish larvae. Adverse side effects caused by ustilotoxin A caused zebrafish larvae to experience growth delays and increased heart rate. Exposure to mycotoxins caused alterations in the expression of 339 transcripts in larvae (Hu et al., 2019). Overall, there are different types ofU.virens found in rice false smut balls that can produce different varieties of ustilotoxins (Lin et al., 2018).
Ustilaginoidea virens
Life Cycle
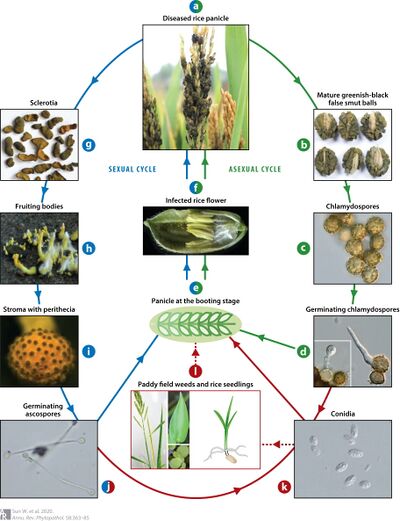
Ustilaginoidea virens, an obligate fungus, has an intricate life cycle within a living host to experience both sexual and asexual reproduction. After infection of the flower organs, mycelia then emerge to cover pistils and form false smut balls. The false smut balls are surrounded by chlamydospores that have thicker walls. The spores are characterized by septae between cells and have irregularly curved and long spikes. Chlamydospores exhibit a high infection rate to initiate infection in rice flowers from late-ripening rice cultivars within the same growing season (Wang et al., 2019). When the infection has matured, the chlamydospores begin to change pigmentation to a dark dark brownish hue on rice spikelets. The Ustilaginoidea virens (U. virens) sclerotia and chlamydospores are spread by air currents and rain splash in paddy fields, which also allows the fungus to survive over the winter. The secondary production of conidia spreads throughout the paddy fields before the seeds of rice are planted and cultivated by farmers. The dormant life cycle of the spores in soil or dead plants becomes a problem as rice false smut disease symptoms can be detected in plants (Wang et al., 2019) The sexual reproduction of Ustilaginoidea virens (U.virens) adopts a heterothallic nature by having two distinct thalli to accomplish successful mating (Zhang et al., 2014).The mating of the fungus involves the compatibility of alleles at the MAT loci. The mating system can be bipolar (unifactorial) or tetrapolar (bifactorial) along with four distinct alleles across two mating type MAT loci. (Yong et al., 2018a). The MAT1 locus is known to have a microsynteny pattern like ascomycetes and the Indian and Chinese strains have common ancestral origin due to having similar gene order and transcription orientation (Huded et al., 2021). Therefore, during sexual reproduction individual isolates must possess MAT 1-1 or MAT 1-2 mating type loci to have successful mating. However, the colder climate conditions in the winter season allow the additional formation of sclerotia formation. In the soil, sclerotia can mature to generate ascospores after their dormancy period, which can last from 2 to 5 months. When it becomes active to establish, sclerotia are then the primary source of infection. The ascospores then infect the flower organs during the booting stage. Perithecia can have numerous asci and 8 immature ascospores. (Yong et al., 2018a).
Nutrient acquisitions
The nutrient acquisition mechanism of Villosiclava virens as a biotrophic fungal pathogen allows it to colonize rice flowers without the formation of appressoria or haustoria. The fungus instead relies on a unique nutrient acquisition strategy by secreting extracellular enzymes that can break down the cell wall of the host plant. Then, the V. virens can absorb nutrients from the surrounding host cells. Therefore, the V.virens can maintain a biotrophic lifestyle to avoid triggering the defense response of the host. The complex relationship between Villosiclava virens and rice spikelets is important to consider in the formation of false smut balls. Infected flowers are unable to produce fertilized ovaries, so the pathogen activates genes associated with grain filling. The stamens are also essential for initial infection to later influence the development of false smut balls. V. virens do not require intact pistils and ovaries to assist with the mechanisms of V. virens nutrient acquisition (Sun et al., 2020; Wang et al., 2019). Therefore, the comprehensive analysis of Villosiclava virens genetic make-up, virulence factor, effector proteins, and nutrient acquisition strategies helps to reveal the pathogenic mechanisms of rice false smut disease (Sun et al., 2020; Wang et al., 2019).
Management of Rice False Smut Disease
To manage rice false smut disease (RSF), breeding resistant varieties of rice cultivars and implementing practices can offer an opportunity to reduce Rice False Smut disease. Therefore, agricultural production can prevent the reduction of 75% of rice yield that is experienced by farmers. In addition, breeding resistant cultivars, early planting of seedlings, and the application of nitrogen fertilizer. It is also important to practice the conservation of tillage and furrow irrigation to prevent the incidence of rice smut disease. The application of fungicide application can also be highly effective against the rice false smut disease, as it offers low toxicity and minimal residue in the fields. Fungicide applications are not environmentally sustainable and not economically affordable for rice farmers. Some recommended fungicides are simeconazole, difenoconazole, and hexaconazole, which have proven to be effective in the reduction of disease incidence. However, it is important to consider the application of fungicide and the type of fungicide used because the fungus is dormant in the soil and can infect flowers during the late booting stage. In the booting stage, the inner flower organs can already be colonized by the pathogen (Wang et al., 2019). 3). Leucine treatment has been shown to provide resistance against rice false smut disease, as it causes the apoptosis of the fungal cells and inhibits the growth of hyphae and spread of the disease in rice paddy fields. Despite leucine treatment promising results in the inhibition and development of rice false smut there needs to be reconsideration on large-scale application and treatment. The application of branch-chained amino acid leucine has been shown to reduce the need for fungicide by 50%, but there needs to be consideration of the environmental changes it might cause in the future, such as soil health (Liu et al., 2023).
References
[Fan, J., Yang, J., Wang, Y., Li, G., Li, Y., Huang, F., & Wang, W. (2016). Current understanding on Villosiclava virens, a unique flower‐infecting fungus causing rice false smut disease. Molecular Plant Pathology, 17(9), 1321–1330. https://doi.org/10.1111/mpp.12362
[Hu, Z., Dang, Y., Liu, C., Zhou, L., & Liu, H. (2019). Acute exposure to ustiloxin A affects growth and development of early life zebrafish, Danio rerio. Chemosphere, 226, 851–857. https://doi.org/10.1016/j.chemosphere.2019.04.002]
[Huded, S., Devanna, P., Prasannakumar, M. K., E., C., Yadav, M., Ngangkham, U., Parivallal, B., Raghavendra, B. T., Channappa, M., Sharma, S. K., & Narayan, K. (2021). Morpho‐molecular and mating‐type locus diversity of Ustilaginoidea virens, an incitant of false smut of rice from Southern parts of India. Journal of Applied Microbiology, 131. https://doi.org/10.1111/jam.15087]
[Jiehua, Q., Shuai, M., Yizhen, D., Shiwen, H., & Yanjun, K. (2019). Ustilaginoidea virens: A Fungus Infects Rice Flower and Threats World Rice Production. Rice Science, 26(4), 199–206. https://doi.org/10.1016/j.rsci.2018.10.007]
[Lin, X., Bian, Y., Mou, R., Cao, Z., Cao, Z., Zhu, Z., & Chen, M. (2018). Isolation, identification, and characterization of Ustilaginoidea virens from rice false smut balls with high ustilotoxin production potential. Journal of Basic Microbiology, 58. https://doi.org/10.1002/jobm.201800167]
[Liu, X., Matsumoto, H., Lv, T., Zhan, C., Fang, H., Pan, Q., Xu, H., Fan, X., Chu, T., Chen, S., Qiao, K., Ma, Y., Sun, L., Wang, Q., & Wang, M. (2023). Phyllosphere microbiome induces host metabolic defense against rice false-smut disease. Nature Microbiology, 8(8), Article 8. https://doi.org/10.1038/s41564-023-01379-x]
[Pieterse, C. M. J., Zamioudis, C., Berendsen, R. L., Weller, D. M., Wees, S. C. M. V., Bakker, P. A. H. M., Nl, C. M. J. P., Nl, C. Z., Nl, R. L. B., Nl, S. V., & Nl, P. A. H. M. B. (2014). Induced Systemic Resistance by Beneficial Microbes. Http://Dx.Doi.Org/10.1146/Annurev-Phyto-082712-102340, 52, 347–375. https://doi.org/10.1146/ANNUREV-PHYTO-082712-102340]
[Sun, W., Fan, J., Fang, A., Li, Y., Tariqjaveed, M., Li, D., Hu, D., & Wang, W.-M. (2020). Ustilaginoidea virens: Insights into an Emerging Rice Pathogen. Annual Review of Phytopathology, 58(1), 363–385. https://doi.org/10.1146/annurev-phyto-010820-012908]
[Wang, W.-M., Fan, J., Jeyakumar, J. M. J., Wang, W.-M., Fan, J., & Jeyakumar, J. M. J. (2019). Rice False Smut: An Increasing Threat to Grain Yield and Quality. In Protecting Rice Grains in the Post-Genomic Era. IntechOpen. https://doi.org/10.5772/intechopen.84862]
[Yu, S., Liu, P., Wang, J., Li, D., Zhao, D., Yang, C., Shi, D., & Sun, W. (2023). Molecular mechanisms of Ustilaginoidea virens pathogenicity and their utilization in disease control. Phytopathology Research, 5(1), 16. https://doi.org/10.1186/s42483-023-00171-3]
[Zhang, Y., Zhang, K., Fang, A., Han, Y., Yang, J., Xue, M., Bao, J., Hu, D., Zhou, B., Sun, X., Li, S., Wen, M., Yao, N., Ma, L.-J., Liu, Y., Zhang, M., Huang, F., Luo, C., Zhou, L., … Sun, W. (2014). Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nature Communications, 5(1), Article 1. https://doi.org/10.1038/ncomms4849 ]
Author
Page is authored by Erica Cortez, a student at Dr. Marc Orbach, University of Arizona.
