User:S4354350
Veillonella Parvula
Morgan Freney, 43543504, Bench B, 23 September 2016
[1]
Classification
Higher order taxa
Kingdom: Bacteria, Phylum: Firmicute, Class: Negativicutes, Order: Selenomonadales, Family: Veillonellanceae, Genus: Veillonella, Species: Parvula. In 2010, the genus Veillionella was emended by Marchandin and colleagues who reclassified all 26 genera of the Selenomonas-Megasphaera-Sporomusa group to the new order Selenomonadales in the new class Negativicutes, and divided into two families; Acidaminococcaceae and Veillonellaceae.[1]
Species
Veillonella parvula; Prévot Te3T [ATCC 10790]
Description and significance
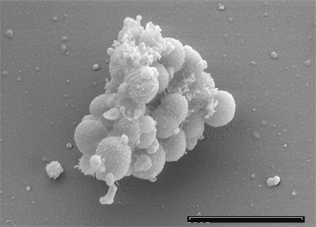
Originally discovered and named as Staphylococcus parvulus by Veillon and Zuber in 1898, Veillonella parvula (V. parvula) was later renamed in 1933 by Prévot [2][3]. A gram-negative species, V. parvula has unusual characteristics that differ from other closely related species in the Firmicutes phylum, of which the majority are gram positive [4]. A non-motile, non-sporulating, obligate anaerobe, V. parvula grow as small cocci typically occurring as diplococci, in short chains or in masses [5]. V. parvula can be identified by its unusual red fluorescence under UV light when grown on trypticase soy agars containing sheep or horse blood [6]. It can also be cultured on multiple other mediums including cysteine-lactose-electrolyte deficient media and hioglycerate broth [7]. V. parvula is unable to utilise glucose or other sources of carbohydrates, instead reducing nitrate and lactate to produce energy [8]. V. parvula can be found in the oral cavity, gastrointestinal, respiratory and genito-urinary tract of homoeothermic vertebrates [9]. Part of the commensal oral flora, V. parvula normally resides in dental plaque and can constitute up to 98% of the cultivable Veillonellae in these sites [4]. It is the only strain of the Veillonellae known to cause oral diseases such as gingivitis. Of significance is the ability of V. parvula to form a biofilm, where it can associate closely with Streptococcus species and participates in opportunistic infections [10][11]. Only rarely has V. parvula been isolated in pure culture. It has been implicated in severe infections in diverse sites such as the lungs, sinuses, liver, heart, bone and central nervous system [4].
Genome structure
The genome of strain Te3T consists of a single circular chromosome 2,132,142 base pairs long with a 38.6% GC content. Of the 1,920 genes, 1,859 have been identified as protein-coding, 61 are RNA genes and there are 15 pseudogenes [4]. The endogenous OK1 plasmid pVJL1 is used as a shuttle vector and is the first genetic tool for Veillonellae transformation [12].
Cell structure and metabolism
V. parvula cocci are approximately 0.3 to 0.5 µm in diameter [4]. Its cell wall consists of the typical gram-negative constituents. Through negative staining, the outer membrane is visibly convoluted, containing different plasmalogens such as plasmenylethanolamine and plasmenylserine which have specific roles in membrane organization, stability and fluidity [13]. Although it is a gram-negative organism possessing lipopolysaccharide (LPS), it is more closely related to gram-positive species like Sporomusa and Megasphaera due to the presence of cadaverine and putrescine, which link covalently to peptidoglycan[13]. V. parvula requires access to these compounds for normal cell growth. V. parvula grows at an optimal temperature between 30-37 degrees Celsius and pH between 6.5-8.0 [4]. The red fluorescence characteristic of V. parvula when grown on brain heart infusion agar containing sheep blood is due to the production of an atypical catalase containing porphyrin [6]. Veillonellae are characterized by an unusual metabolism using methylmalonyl-CoA decarboxylase to convert the free energy derived from decarboxylation reactions into an electrochemical gradient of sodium ions [4]. Veillonellae cannot utilise energy sources such as carbohydrates or polyols due to lacking hexokinase [8]. Instead fermenting pyruvate, lactate, malate, fumarate, and oxaloacetate to produce propionic and acetic acid [4]. The presence of a functional glycolic system missing only hexokinase suggests that the pathway could instead be a viable for the production of glucose-6-phosphate through gluconeogenesis. Interestingly, while V. parvula cannot grow on succinate as a sole carbon source, it can decarboxylate succinate during fermentation of lactate or malate, and utilise succinate as a co-factor for enhancing growth rate or energy conservation[4].
Ecology
V. parvula is a moderate anaerobe able to tolerate oxygen concentrations above 0.5% [15]. Typically found in the oral cavity, genito-urinary, respiratory, and intestinal tracts of humans, dental plaque, buccal mucosa, and the tongue are the main ecological niches of V. parvula [10]. The formation of plaque provides favourable anaerobic conditions for V. parvula. V. parvula has been found to make up almost 10% of early supra and subgingival plaque colonisers[10]. While V. parvula cannot adhere to surfaces itself due to lacking flagella and adhesion structures. The bacterium circumvents this problem by attaching to specific surface structures present on other cells, mediated by lectin-carbohydrate interactions[10]. The intra-oral distribution of Veillonellae is therefore dependent upon their binding to specific members of the oral flora. By forming intergeneric co-aggregates with other bacteria which occur in the same ecological niche, V. parvula plays a critical role in establishing the bacterial ecology of the oral cavity. V. parvula utilizes the metabolic end products of co-existing carbohydrate-fermenting bacteria, which forms the basis of a functional community providing nutrients and protection[11]. While, host interactions with V. parvula usually occur indirectly through association with other pathogenic bacterial species. It has been suggested that, since Veillonella metabolize lactate and succinate, they may be more abundant in caries lesions, and their metabolism may enhance the caries process [4].
Pathology
V. parvula is rarely pathogenic in humans. Opportunistic infections caused by V. parvula are most frequently reported as osteomyelitis and endocarditis [16]. Occassionally, cases of severe bacteraemia are reported[16]. V. parvula has often been identified in cases of severe early childhood caries, in intracranial abscesses, in sinusitis and in dentinal tubules[10]. Less frequent are cases of deep neck infections, chronic maxillary sinusitis, meningitis, vaginitis, tonsillitis, discitis, prosthetic joint infection and pneumonitis[17][18][19]. Factors that increase the susceptibility to V. parvula infection include use of intravenous drugs, immune deficiency, premature birth and periodontal disease[17]. Although, the pathogenic role of V. parvula in oral infections has not yet been fully elucidated. It has been suggested that this organism may facilitate the succession of species in developing oral biofilms, hence, the pathogenicity of other bacteria[10]. V. parvula? is susceptible to numerous antimicrobials including; ampicillin, tazobactam, cefoxitin, cefotetan, clindamycin, ceftriaxone, imipenem, meropenem, bacitracin, metronidazole, remoplanin, trimethoprim and vanomycin. Whereas, resistance to amoxycillin and penicillin has been observed[20]. Dual species biofilms containing V. parvula and Streptococcus mutans (S. mutans) have enhanced resistance to chlorhexidine than either species alone[20]. V. parvula is able to change the gene expression of S. mutans, hence altering its physiology. In this way, V. parvula aids other more pathogenic bacteria such as S. mutans to cause infections such as periodontal disease. V. parvula also contains two chemically and immunologically distinguishable polysaccharide-lipid complexes which are known virulence factors [4].
Application to biotechnology
At present, V. parvula is not recognised as having any potential biotechnological applications. In an effort to uncover the biology of the Veillonella genus, researchers are developing methods for genetic manipulation. The endogenous OK1 plasmid pVJL1 has been engineered as a shuttle vector and is the first genetic tool for Veillonellae[12]. Only recently was transformation achieved by obtaining spontaneous mutants carrying a K43N substitution in the RpsL protein that conferred streptomycin resistance. The ability to transform V. parvula through the use of the mutated rpsL gene as a selection marker was determined to be inefficient for routine use as an established transformation protocol[21].
Treatment of V. parvula infections is usually with the 5-nitroimidazole antibiotic metronidazole [4]. The mechanism of action involves the formation of cytotoxic free-radicals when reduced by pyruvate-ferrodoxin oxidoreductase, which degrades DNA. Combinational drugs are necessary to treat infection with V. parvula due to resistance and the presence of β-lactamase. Often vancomycin, ceftriaxone and metronidazole are used[20].
Current research
A recent study established a novel one-step PCR method to effectively detect Veillonella species in human oral cavities[22]. This report investigated the distribution and frequency of Veillonella species in tongue biofilms in children and found that Veillonella species may be an index of oral hygiene status. With those subjects found to have poor oral hygiene containing a larger abundance of Veillonellae.
A Japanese news report also recently proposed the discovery of a new Veillonellaa species isolated from human tongue biofilms, named Veillonella tobetsuensis[23].
Multiple studies investigating pathogens implicated in periodontal diseases have suggested that research should be focus more on multispecies biofilms when testing of the efficacy of antimicrobials[24][20].
References
1. [1] Marchandin, H., Teyssier, C., Campos, J., Jean-Pierre, H., Roger, F., Gay, B., Carlier, J. and Jumas-Bilak, E. (2009). Negativicoccus succinicivorans gen. nov., sp. nov., isolated from human clinical samples, emended description of the family Veillonellaceae and description of Negativicutes classis nov., Selenomonadales ord. nov. and Acidaminococcaceae fam. nov. in the bacterial phylum Firmicutes. International Journal of Systemic and Evolutionary Microbiology, 60(6), pp.1271-1279.
2. Veillon A, Zuber MM. Recherches sur quelques microbes strictement anaérobies et leur rôle en pathologie. Arch Med Exp 1898; 10:517-545
3. Prévot AR. Études de systématique bactérienne. I: Lois générale. II: Cocci anaérobies. Ann Sci Nat Bot 1933; 15:23-260
4. [2] Aujoulat, F., Bouvet, P., Jumas-Bilak, E., Jean-Pierre, H. and Marchandin, H. (2014). Veillonella seminalis sp. nov., a novel anaerobic Gram-stain-negative coccus from human clinical samples, and emended description of the genus Veillonella. International journal of systematic and evolutionary microbiology, 64(Pt 10), pp.3526-3531.
5. [3] Gronow, S., Welnitz, S., Lapidus, A., Nolan, M., Ivanova, N., & Glavina Del Rio, T. et al. (2010). Complete genome sequence of Veillonella parvula type strain (Te3T). Standards In Genomic Sciences, 2(1), 57-65. http://dx.doi.org/10.4056/sigs.521107.
6. [4] Brazier, J. and Riley, T. (1988). UV Red Fluorescence of Veillonella spp. Journal of Clinical Microbiology, 26(2), pp.383-384.
7. [5] Berenger, B., Chui, L., Borkent, A. and Lee, M. (2015). Anaerobic urinary tract infection caused by Veillonella parvula identified using cystine-lactose-electrolyte deficient media and matrix-assisted laser desorption ionization-time of flight mass spectrometry. ID Cases, 2(2), pp.44-46.
8. [6] Bishop, F., Rogosa, M. and Krichevsky, M. (1965). Truncated Glycolytic System in Veillonella. Journal of Bacteriology, 90(1), p.164.
9. [7]Marchandin, H., Teyssier, C., Campos, J., Jean-Pierre, H., Roger, F., Gay, B., Carlier, J. and Jumas-Bilak, E. (2009). Negativicoccus succinicivorans gen. nov., sp. nov., isolated from human clinical samples, emended description of the family Veillonellaceae and description of Negativicutes classis nov., Selenomonadales ord. nov. and Acidaminococcaceae fam. nov. in the bacterial phylum Firmicutes. International journal of systematic and evolutionary microbiology, 60(6), pp.1271-1279.
10. [8] Moore, L., Hughes, C., Kolenbrander, P. and Andersen, R. (1988). Coaggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Applied and Environmental Microbiology, 54(8), p.1957.
11. [9] Palmer, R., Diaz, P. and Kolenbrander, P. (2006). Rapid Succession within the Veillonella Population of a Developing Human Oral Biofilm In Situ. Journal of Bacteriology, 188(11), pp.4117-4124.
12. [10] Liu, J., Xie, Z., Merritt, J. and Qi, F. (2012). Establishment of a Tractable Genetic Transformation System in Veillonella spp. Applied and Environmental Microbiology, 78(9), pp.3488-3491.
13. [11] Stackebrandt, E., Pohla, H., Kroppenstedt, R., Hippe, H. and Woese, C. (1985). 16S rRNA analysis of Sporomusa, selenomonas, and Megasphaera: on the phylogenetic origin of Gram-positive Eubacteria. Archives of Microbiology, 143(3), pp.270-276.
14. [12] Rogosa, M. (1971). Transfer of Veillonella Prevot and Acidaminococcus Rogosa from Neisseriaceae to Veillonellaceae fam. nov., and the Inclusion of Megasphaera Rogosa in Veillonellaceae. International Journal of Systematic Bacteriology, 21(3), pp.231-233.
15. [13] Holý, O. and Chmelař, D. (2012). Oxygen tolerance in anaerobic pathogenic bacteria. Folia Microbiol, 57(5), pp.443-446.
16. [14] Marriott, D., Stark, D. and Harkness, J. (2006). Veillonella parvula Discitis and Secondary Bacteremia: A Rare Infection Complicating Endoscopy and Colonoscopy? Journal of Clinical Microbiology, 45(2), pp.672-674.
17. [15] Shah, A., Panjabi, C., Nair, V., Chaudhry, R. and Thukral, S. (2008). Veillonella as a cause of chronic anaerobic pneumonitis. International Journal of Infectious Diseases, 12(6), pp.e115-e117.
18. [16] Spiegel, C. (1991). Bacterial vaginosis. Clinical Microbiology Reviews, 4(4), p.485.
19. [17] Bhatti, M. and Frank, M. (2000). Veillonella parvula Meningitis: Case Report and Review of Veillonella Infections. Clinical Infectious Diseases, 31(3), pp.839-840.
20. [18]Luppens, S., Kara, D., Bandounas, L., Jonker, M., Wittink, F., Bruning, O., Breit, T., ten Cate, J. and Crielaard, W. (2008). Effect of Veillonella parvula on the antimicrobial resistance and gene expression of Streptococcus mutans grown in a dual-species biofilm. Oral Microbiology and Immunology, 23(3), pp.183-189.
21. [19] Liu, J., Merritt, J. and Qi, F. (2011). Genetic transformation of Veillonella parvula. FEMS Microbiology Letters, 322(2), pp.138-144.
22. [20] Mashima, I., Theodorea, C., Thaweboon, B., Thaweboon, S. and Nakazawa, F. (2016). Identification of Veillonella Species in the Tongue Biofilm by Using a Novel One-Step Polymerase Chain Reaction Method. PLOS ONE, 11(6), p.e0157516.
23. [21] Carrouel, F., Viennot, S., Santamaria, J., Veber, P. and Bourgeois, D. (2016). Quantitative Molecular Detection of 19 Major Pathogens in the Interdental Biofilm of Periodontally Healthy Young Adults. Front. Microbiol., 7.
24. [22] Mashima, I., Kamaguchi, A., Miyakawa, H. and Nakazawa, F. (2012). Veillonella tobetsuensis sp. nov., an anaerobic, Gram-negative coccus isolated from human tongue biofilms. International journal of systematic and evolutionary microbiology, 63(Pt 4), pp.1443-1449.
- ↑ MICR3004
This page is written by Morgan Freney (43543504) for the MICR3004 course, Semester 2, 2016