User:S4355920
Name: Amy Pham
Bench ID: C
Date: 21/09/2016
MICR3004
Classification
Higher order taxa
Bacteria – Bacteroidetes – Bacteroidia – Bacteroidales – Porphyromonadaceae - Porphyromonas - P. gingivalis
Species
Species: Porphyromonas gingivalis
Strain: 2561 = ATCC 33277 = CCUG 25893 = CCUG 25928 = CIP 103683 = DSM 20709 = JCM 12257 = NCTC 11834
Description and significance
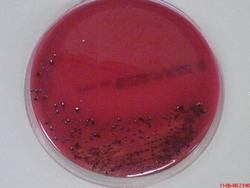
Porphyromonas gingivalis is an obligately aerobic, gram-negative bacterium belonging to the phylum Bacteroidetes [2]. Characterised by its rod shaped morphology, it is a non-spore bearing and non-motile bacterium most commonly found inhabiting the oral cavity [2]. Recognised as an opportunistic pathogen, P. gingivalis is capable of living in commensal harmony with the host [3]. Termed as a pathobiont, the bacterium can cause episodes of diseases when a change in the ecological balance of the periodontal microenvironment transpires [4] [5]. Although the bacterium is capable of existing as a commensal organism, certain strains are known to be more virulent and pathogenic than others [3]. Virulent strains include, W83, W50, ATCC 49417 and A7A1 [6] [7]. Avirulent strains include ATCC 381, 33277 and 23A4 [6] [7]. In vitro studies of the bacterium have found cells cultured in broth with a size range from 0.5 by 1 to 2 μm [2]. Cells grown on a solid media showed coccobacilli or very short rod structures [2]. On blood agar plates, the bacterium forms black-pigmented colonies and are predominately smooth, shiny and convex with a diameter between 1 to 2 mm [2] [8].
Typically found in the oral cavity of individuals, P. gingivalis has been implicated with periodontal diseases, most commonly associated with chronic periodontitis [3]. A report from the Centres for Disease Control and Prevention (CDC) recorded 47. 2 % of adults in the United Stated aged 30 years or older have experienced some form of periodontal disease [9]. In light of this information, recent studies have also reported P. gingivalis to be associated with systematic diseases, including cardiovascular diseases, rheumatoid arthritis and decreased kidney function [10]. Studies underlying the molecular mechanisms behind bacterial pathogenesis are key to design effective treatments. Consequently reducing the potential development of inflammatory diseases that arise as a secondary consequence to periodontitis.
Genome structure
P. gingivalis strain W88 has a circular chromosome made up of 2 343 479 bp’s [11]. On average the guanine and cytosine content make up approximately 48.3 % of the genome [11].The circular chromosome encodes 1909 protein genes and 65 RNA genes [11]. This includes 4 ribosomal operons (rrn, 5S rRNA-23S rRNA-tRNAAla-tRNAIle-16S rRNA) and 53tRNA genes[11]. Interestingly the number of rrn operons and tRNA genes in strain W83 are identical to those of an avirulent strain counterpart ATCC 33277 [7] [11]. The extensive rearrangement between the two strains through the introduction of mobile elements inevitably altered the virulence of the bacterium [11].
The genome of W83 is composed predominately composed of ORF (85%) [11]. Of the1,990 ORF’s encoded, 1075 have detectable biological roles [11]. Of the remaining ORF, 184 were categorised as a conserved hypothetical protein or conserved domain protein, 208 had to known function, and 523 encoded hypothetical proteins [11].
Cell structure and metabolism
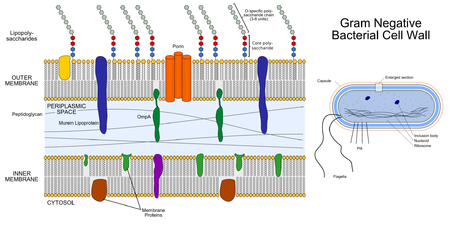
Cell Wall: P. gingivalis is an obligately aerobic, non-motile gram-negative bacterium [2]. The cell wall is characterised by three distinct layers, including two membranous structures known as the inner membrane (IM) and the outer membrane (OM) [12]. Connecting the two layers is a gel like structure known as the periplasm and a thin layer of peptidoglycan [12]. The IM and OM possess a trilamellar structure composed of phospholipids [12]. Distributed along the outer membrane are lipoproteins and lipopolysaccharides (LPS), which serve as an anchor for lipids [12]. Chemically LPS is composed of three subunits, the O specific polysaccharide chain, the core and lipid A [12].
Fimbriae: Protruding the outer membrane of the cell wall, thin proteinaceous surface appendages aid and mediate bacterial attachment to the host [8]. Approximately 25 μm long these structures have a robust ability to interact with salivary proteins, epithelial cells, extracellular matrix proteins and the fibroblasts of the host [8]. Two distinct fimbriae types are displayed on the cell surfaces of the bacteria, known as FimA and Mfa protein [8]. These surface structures are proposed to have a role in the progression of periodontal inflammatory reactions. Six genotypes of FimA structures exist (type I-V and Ib), ranging from 40.5 to 49kDa in size [8]. Strain W83 is classed under type IV and are poorly fimbriated whereas strain ATCC 33277 is an abundantly fimbriated type I strain [8]. The progression of chronic periodontitis is most closely associated with type II strains followed by type IV [8].
Biofilm formation: The bacterium colonises the oral cavity by forming a complex biofilm known as plaque [13]. P. gingivalis is recognised as a secondary or late coloniser and require antecedent organisms to form the necessary environmental conditions for growth [8]. Upon contact the bacterium must resist the plethora of host responses working against bacterial colonisation [13]. Host factors are known to include mechanical shearing produced from the force of the tongue, saliva and gingival crevicular fluid flow [13]. Successful colonisers must therefore possess a diverse repertoire of virulence factors to overcome host defences [13].
Motility: Non-motile [3]
Metabolic Functions: P. gingivalis is dependent on nitrogenous substrates for energy production [13]. Despite the nitrogenous compounds present in the oral cavity, the bacterium has a limited ability to ferment free amino acids [13]. Aspartic acid and asparagine are among the few which can be metabolised to yield succinate.
Ecology
Anaerobe: In the literature, P. gingivalis was previously regarded as a strictly anaerobic bacterium [14]. However current understanding of its genome composition indicates the presence of an oxygen metabolism pathway [14]. Oxidative stress mechanisms are most likely employed to cope with the brief exposure to oxygen on the route towards peridontal pockets. Analysis of genome composition, revealed the presence of aerobic respiration genes (cydA and cydB), encoding the haem protein cytochrome bd oxidase [14].
Habitat: P. gingivalis is a natural inhabitant of the oral microbiome and is typically found residing in the subgingival sulcus of the oral cavity [5] [15]. The bacterium can also be found along the cheek, gingiva and tongue [3]. Although colonisation of the gingival crevice and periodontal pocket are typically associated with infection, colonisation of remote surfaces can also lead to disease [3]. Recognised as a secondary or late coloniser, attachment to antecedent organisms is typically evident [8]. Primary organisms provide a foundation for attachment and form the necessary environmental conditions for growth. Such as supplying growth substrates and decreasing oxygen tension [3] [8] Attachment to early plaque organisms includes species of oral Streptococci (Streptococcus gordonii, S. sanguis, S. oralis, S. mitis, and S. crista) and Actinomyces naeslundii [3] [16] [17] [18] [19].
Microbe/Host Interaction: P. gingivalis is as an opportunistic pathogen and is capable of living in commensal harmony with the host [5]. Termed as a pathobiont, the bacterium can cause episodes of diseases when a change in the ecological balance of the periodontal microenvironment arises [3] [5].
Pathology
Periodontal Disease: Periodontal disease is an infection of the supporting structures of the periodontium [8]. Initial stages of periodontal disease are associated with gingivitis [8]. Gingivitis is an inflammation of the gum tissues initiated by the formation of plaque[8]. It is characterised by swelling, redness and bleeding of the tissues [8]. If left untreated inflammation of the periodontal tissues can lead to the destruction of the alveolar bone and periodontal ligament [8]. P. Gingivalis is the major etiologic agent causing the majority of periodontitis cases [20]. It has also been associated with systematic diseases, including cardiovascular diseases, rheumatoid arthritis and decreased kidney function [10].
Periodontitis can be classified into three different classes depending on its severity [20]. Aggressive periodontitis is accompanied by the induction of pro-inflammatory cytokines, IL1-β and IL-6 produced by CD4+ T helper cells [20]. Destructive periodontitis is associated with Th1 and Th17 immune response and chronic periodontitis is associated with primarily Th17 pathways [20].
Properties and Pathogenicity of Porphyromonas gingivalis:
The length of infection caused by P. gingivalis is partly dependent on the bacteria’s modulation of host responses [20]. An arsenal of virulence factors is exploited to ensure the long-term survival of the bacterium within the oral cavity [20].
The virulence factors produced by P. gingivalis include the following: [3] [8] [20].
- Enzymes (hyaluronidase, chondroitin and sulfatases)
- Capsule
- Lipopolysaccharide
- Fimbriae
- Exopolysaccharide
- Outer membrane proteins
- Collagenase
- Trypsin-like protease
- Gelatinase
- Aminopeptidase
- Haemagglutinins
- Gingipains
Expression of these virulence factors can lead to destruction of the periodontal tissue, induction of host response and inhibition of host protective mechanisms [3].
Upon entry into the oral cavity P. gingivalis interacts with host epithelial cells [3]. Epithelial cells are natural sensors of microbial invasion, generating and transmitting signals to immune cells in the periodontal tissues [3]. Following the adherence to host cells, P. gingivalis is internalised into a membrane bound vesicle [20]. Once established, the bacterium forms a replicative niche by activating cellular autophagy and suppressing apoptosis [20]. The bacterial plaque formed by P. gingivalis results in inflammation of the periodontal tissues, resulting in epithelial migration and the destruction of the alveolar bone and periodontal ligament [8]. This is induced by the induction of host immune responses, releasing pro-inflammatory cytokines such as IL-1β and IL-6 [20]. Consequently a periodontal pocket is produced, opening up the opportunity for sub gingival plaque formation [8].
Application to biotechnology
The development of novel approaches to combat bacterial infections has increased due to the ongoing emergence and documentation of resistance to antimicrobial agents. The power of biotechnology has been a major contributor to recent advances in drug development [21]. Specifically, in the new generation of antimicrobial agents whose target is to inhibit the function and expression of major virulence factors. Proteases of P. gingivalis are key factors in the pathogenesis and progression of periodontitis, in particular R- and K- gingipains [3] [8] [20] [21]. These proteases are known to deregulate the inflammatory response and permit the evasion host defences [21]. In vitro studies have shown broad, non-specific inhibitors of protease to increase immune responses. In particular complement mediated opsonophagocytosis [21]. Isogenic protease (rgpA, rgpA and kgp) has also been targets to decrease the bacterial nutrient uptake and cell surface proteolytic processing of the bacterium [21]. Therefore extensive research and time has been invested into producing specific protease inhibitors to control the bacterium.
Current research
Gingipains from the Periodontal Pathogen Porphyromonas gingivalis Play a Significant Role in Regulation of Angiopoietin 1 and Angiopoietin 2 in Human Aortic Smooth Muscle Cells:
Although P. gingivalis is predominately associated with chronic periodontitis, recent studies have found the bacterium to be associated with systematic diseases, in particular the progression of cardiovascular diseases [10] [22]. Conducted by Zhang et al, the aim of the study was to anaylse the effects of a P. gingivalis infection on the gene expression of two ligands known to play an important role in the development of inflammatory diseases [22]. This included Angpt1 and Angpt2 in human aortic smooth muscle cells (AoSMCs). To identify the effects of the bacterium on muscles, samples were infected with the pathogen and analysed[22]. The results established a direct casual link between the periodontal bacterium and cardiovascular diseases. Infection caused by the bacterium was found to down regulate anti-inflammatory gene Angpt1 and up regulate pro-inflammatory gene Angpt2 [22].
Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer:
Another recent study looking into the role of P. gingivalis has further demonstrated a casual relationship, which was previously unknown. In the study conducted by Goa et al., an association with P. gingivalis and the progression of esophageal squamous cell carcinoma was established [23]. To examine the presence of P. gingivalis in esophageal mucosa an immunohistochemistry assay was performed targeting gingipains Kgp [23]. qRT-PCR confirmed the presence of P. gingivalis 16S rDNA. 61% of cancerous tissue was detected to harbor P. gingivalis, compared to 0% detected in normal esophageal mucosa [23]. Although the exact role of P. gingivalis in esophageal cancer has not yet been confirmed, the findings demonstrated that p. gingivalis is found to harbor environments outside of the oral cavity.
References
8. [1]
9. [2]
This page is written by Amy Pham for the MICR3004 course, Semester 2, 2016