User talk:CrowJ
By: Jack Crow
General Information
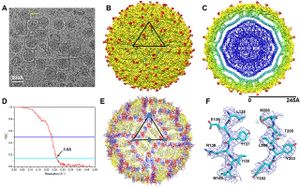
Zika virus (ZIKV) is a member of the virus family Flaviviridae and the genus Flavivirus.[1] It is spread by Aedes mosquitoes, for instance A. aegypti. The virus was first isolated in the Zika Forest of Uganda, in 1947.[2] Zika virus is related to other Flaviviruses: dengue, yellow fever, Japanese encephalitis, and West Nile viruses.[3] The virus causes a fever, often with none or only mild symptoms, and go unrecognized in the infected host. In 2013, the virus spread eastward across the Pacific Ocean to French Polynesia, New Caledonia, the Cook Islands, and Easter Island, and in 2015 to Mexico, Central America, the Caribbean, and South America, notably in Brazil. As of 2016, the illness cannot be prevented by medications or vaccines.[4] Zika is thought to spread from an infected pregnant women to the baby. The CDC has linked infant infection with microcephaly and other severe brain problems. Zika infections in adults has been shown to cause Guillain-Barré syndrome.[5] The purpose of this paper is to examine the evolution, the development of virulence in the virus and the link between severe autoimmune-neurological syndrome in infected human hosts and the virus.
Diagnosis and Vaccine Development
The Zika virus is believed to interact with human immune system similarly to other flavivirus family viruses. The human immune response to flaviviruses is a complicated relationship. These viruses can be both beneficial and pathogenic, and there may be interactions between different viruses and phenotypes of viruses.[6] Building a comprehensize understanding of the disease, and the continuum of possible effects of the disease, is essential in developing a functional characterization of Zika virus infection.
Difficulties arise in developing strong diagnostic standards. Confirmation of virus presence in potential hosts, is typically done in laboratory settings, “by testing serum for viral nucleic acid of virus-specific IgM and IgG antibodies.”[7] Huang et al. (2016) discuss the difficulty in evaluating potential infected hosts more than seven days after the initial acquisition of the virus. After seven days, “negative RT-PCR results… do not rule out flavivirus infection.”[8] Furthermore, Huang et al. (2016) report cross reactivity of antibodies between Zika and Dengue viruses. Diagnostics, and vaccine development, are further complicated by possible interactions between Zika and Chikungunya viruses. Faria et al. (2016) report that pathogenesis may be enhanced by interaction between these two viruses.[9] Therefore a positive diagnosis of Zika virus infection may not always necessarily confer the severe adverse health effects currently associated with the Zika virus.
Vaccine development is still in the experimental phases of development. Modification of existing flavivirus vaccine may provide the most promising pathway for Zika virus vaccine development. Dengvaxia, the first registered Dengue virus vaccine, is in stage III of clinical drug development, and is approved for use in infected individuals between the ages of nine and forty-five in endemic areas.[10] Furthermore, vaccines against yellow fever and Japanese encephalitis have been available for more than twenty years.[11] However, Huang et al. (2016) caution against over-dependence on existing flavivirus vaccines, citing positive and or negative interference in patients with flavivirus immunity and of vaccine-induced development of Guillain-Barre syndrome.[12]
Spread of the Virus and Characterization of Mutations
The virus is believed to have originated from central Africa, spread to Asia, on to the Pacific Islands,from there to Brazil and from Brazil north to Central and North America (fig. 2). Faria et al. estimate that American serotypes of the Zika virus are mutating at a rate from 0.98 to 0.00106 nucleotide substitutions / site / year. [13] They suggest this mutation rate is consistent with mutation rates of previous epidemics, and is higher than mutation rates in other flaviviruses. Consequently, it is likely that the American serotypes of the Zika virus are hypermutable. Faria et al (2016) discuss three hypotheses for the introduction of the virus to Brazil: during the 2014 World Cup soccer tournament (June and July, 2014), during the Va’a canoe race held in Rio de Janeiro (August, 2014) and during the 2013 Confederations Cup soccer tournament (June, 2013). The investigators state that their molecular clock analyses suggest that the introduction of the virus to Brazil predates the World Cup and Va’a canoe race, but that the Confederations Cup ended before the symptoms associated with the more pathogenic serotypes were reported in French Polynesia
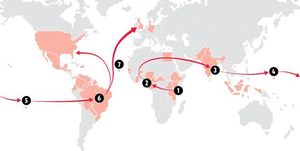
Potential Spread of the Virus and Disease Prevention
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ http://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0004530
- ↑ http://www.jgid.org/article.asp?issn=0974-777X;year=2016;volume=8;issue=1;spage=3;epage=15;aulast=Sikka
- ↑ http://www.jgid.org/article.asp?issn=0974-777X;year=2016;volume=8;issue=1;spage=3;epage=15;aulast=Sikka
- ↑ http://www.cdc.gov/zika/symptoms/
- ↑ http://apps.who.int/iris/bitstream/10665/204961/1/zikasitrep_7Apr2016_eng.pdf?ua=1
- ↑ http://www.nejm.org/doi/full/10.1056/NEJMp1603734
- ↑ http://www.nejm.org/doi/full/10.1056/NEJMp1603734
- ↑ http://www.nejm.org/doi/full/10.1056/NEJMp1603734
- ↑ http://science.sciencemag.org/content/early/2016/03/23/science.aaf5036
- ↑ http://www.who.int/immunization/research/development/dengue_vaccines/en/
- ↑ http://www.nejm.org/doi/full/10.1056/NEJMp1603734
- ↑ http://www.nejm.org/doi/full/10.1056/NEJMp1603734
- ↑ http://science.sciencemag.org/content/early/2016/03/23/science.aaf5036
- ↑ http://www.independent.co.uk/life-style/health-and-families/health-news/zika-how-virus-spread-around-world-a6839101.html
- ↑ http://www.vox.com/2016/4/6/11348908/zika-science
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
