Variovorax paradoxus
Classification
Domain: Bacteria; Phylum: Proteobacteria; Class: Betaproteobacteria; Order: Burkholderiales; Family: Comamonadaceae; Genus: Variovorax (30)
Species
|
NCBI: Taxonomy |
Variovorax paradoxus
Description and Significance
Habitat and Occurrence in Environment
V. paradoxus is commonly found in the rhizosphere, which is the area between the roots and soil of plants (1). It is in symbiotic relationships with nitrogen-fixing microbes, plants, and photosynthetic epionts (8).
Significance and Description
The name Variovorax paradoxus refers to the ability to devour (metabolize) a large variety of substrates, as well as its almost paradoxal organotrophic and chemolithotrophic metabolism (26). The degradation of AHL by V. paradoxus promotes a synergistic relationship between the bacterium and the plant. AHL is a compound involved in bacterial quorum sensing; as V. paradoxus degrades this compound, it promotes plant growth by destroying harmful bacteria (8). V. paradoxus is coined as a PGPR, or a plant-growth promoting rhizobacteria, as the bacterium’s metabolic diversity promotes plant growth by destroying harmful environmental pollutants (8). In addition to other Variovorax species, V. paradoxus has also been found to be one of the most important bacterial players in cycling sulfonate-sulfur. When V. paradoxus strains are cultured alongside methanotrophs, the bacterium shows an affinity for degrading methane, a toxic environmental pollutant (10).
Genome Structure
Four strains of V. paradoxus have been sequenced fully. The S110 strain was found to have two circular chromosomes, the first with 5,626,355 bp and the second with 1,128,646 bp (8). The G/C content of the S110 strain is 67.5%. There was found to be 6,279 predicted protein coding genes, 4,557 of which have predicted roles based on similarity searches (8). The EPS strain of V. paradoxus was found to have one circular chromosome with 6,550,056 bp (9). The G/C content of the EPS strain is 66.48% (9). There was found to be 6,020 predicted protein coding genes for this strain (9). The TBEA6 strain of V. paradoxus was found to have around 7.2 Mbp and 6,852 predicted protein coding genes (28). The B4 V. paradoxus strain was found to have two circular chromosomes, the first with 5,795,261 bp and the second with 1,353,255 bp; 6,753 genes were identified to be protein coding (4).
Cell Structure, Metabolism and Life Cycle
Cell Structure
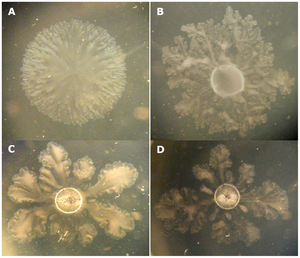
V. paradoxus can appear as straight or curved rods, and have a singular flagella (26). The bacterium have a yellow color resulting from a carotenoid pigment, or a pigment present in photosynthetic bacteria to protect against light damage (26, 14, 15) . When cultured, V. paradoxus can develop as a singular bacterium or grouped in pairs (26). The bacterium can range from 0.5 to 0.6 micrometers or 1.2 to 3.0 micrometers, depending on the culture (26). V. paradoxus is a gram negative bacterium, meaning that their cell wall includes a lipopolysaccharide (LPS) bilayer (12). Growth conditions for V. paradoxus culture vary depending on strain-type, but most cultures can be grown in a 30℃ environment (14).
Metabolism
The bacterium metabolizes aerobically with some strains demonstrating lithoautotrophy and others chemoorganotrophy (29). V. paradoxus can participate in the degradation of both biogenic and anthropogenic compounds (19). The bacterium catabolizes by degrading pollutants like sulfur and amino acids that are detrimental to the environment (19). V. paradoxus S110 strain degrades pesticides and S-metabolites by metabolizing AHL, or Acyl Homoserine Lactone (8, 24). V. paradoxus EPS strain has similar properties, exemplifying the ability to metabolize AHL (9). V. paradoxus B4 strain metabolizes anthropogenic compound MS (mercaptosuccinate), using MS as a source of carbon (4). V. paradoxus TBEA6 degrades thiodipropionic acid (TDP) as a source of sulfur and energy (28). Overall, V. paradoxus strains display very diverse metabolisms, capable of degrading a wide range of chemicals.
Ecology and Pathogenesis
V. paradoxus is a ubiquitous soil microbe naturally found within the rhizosphere, and is a member of a group known as the plant growth-promoting rhizobacteria (PGPR) (29). The most consequential ecological implications of V. paradoxus stem from its behaviors as a PGPR as well as its very flexible metabolism. Members of this group are known to have various beneficial effects on plant growth, such as production of beneficial metabolites for plant symbionts or consumption of harmful chemicals such as toxic contaminants. Certain strains of Variovorax have been seen to have effects such as increasing stress tolerance and resistance to diseases in a mutualistic relationship with plants. V. paradoxus strains could be particularly apt at their beneficial behaviors with plants due to their endobiotic symbioses, as endobionts interact with their hosts even more closely than ectosymbionts (17).
Effects on Plant Growth
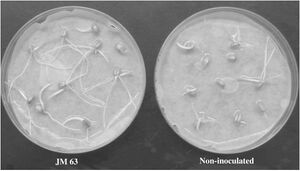
V. paradoxus is able to utilize hydrogen gas as an electron donor. Hydrogen gas is a byproduct of nitrogen-fixation within the rhizosphere.V. paradoxus strains that oxidize hydrogen gas exist in syntrophic relationship with these N-fixing microbes as they increase the ability of these microbes to perform nitrogen-fixation and thus aid these microbes as well as their mutualist plant partners. Through this process, V. paradoxus was found to result in increases in plant root and overall growth (29). This can be observed in the image of plant roots shown below.
Increased Tolerance to Environmental Stressors
A common result of environmental stressors on plants, such as drought or exposure to high concentrations of heavy metals, is the stimulation of ethylene production, which inhibits crop growth and crop yields (5). One such relationship has been documented between V. paradoxus and the indian mustard plant, in which V. paradoxus was found to promote root growth of the plant in a cadmium (heavy metal) rich environment while utilizing 1-aminocyclopropane-1-carboxylate (ACC), the precursor of the plant hormone ethylene (2). This activity is made possible by the presence of ACC deaminase within Variovorax strains, which catabolizes ACC into ammonia and alpha-ketobutyrate. These metabolites can then serve as sources of carbon and nitrogen for V. paradoxus. This mutualistic relationship is beneficial for both the bacterium as well as the indian mustard plant, as the V. paradoxus strain 5C-2 utilizes a plentiful N source and thereby inhibits ethylene production for the benefit of the plant, which positively regulates growth as ethylene accumulation is negatively affected. The ability of Variovorax to behave in this manner relies upon the resistance of these bacteria to heavy metals within the environment in this study (2).
Another possible increase to stress tolerance that V. paradoxus exerts to plants is increased resistance to drought and water stress. In a similar mechanism as with heavy metal stress, V. paradoxus can interfere with ethylene production in order to increase drought tolerance within plants. One study found that within an experimental group of pea plants treated with V. paradoxus, the crop yield was approximately 50% greater compared to the pea plants in drought conditions without addition of V. paradoxus. Furthermore, this same experiment found that V. paradoxus inoculations increased total and specific root nodule numbers, providing evidence that V. paradoxus may also be beneficial due to the synergistic effect of preventing drought-induced nodule inhibition and therefore increasing the ability of rhizobia to continue fixing nitrogen for the benefit of the plants (3). It is likely that ACC deaminase coupled with hydrogen oxidation can lead to the most intense plant-growth promoting behaviors.
Sulfur Compound Metabolism
Along with possible beneficial symbioses such as maintaining root nodules within plants, V. paradoxus strains can also increase the ability of the plant host to more effectively uptake nutrients such as inorganic sulfur. Variovorax has the ability to metabolize sulfonates due to the activity of oxidoreductase AsfA, which through metabolizing environmental sulfur compounds frees up inorganic sulfur for use by plants (2). Another related class of molecules that V. paradoxus can metabolize is that of sulfolane (tetrahydrothiophene-1,1-dioxide). These molecules are byproducts of the process by which natural gas is sweetened, and are classified as high risks for contaminating groundwater. V. paradoxus is able to metabolize these molecules into sulfates, which can also potentially be used by other organisms or by plants within the rhizosphere (7).
Degradation of Acyl-homoserine Lactones
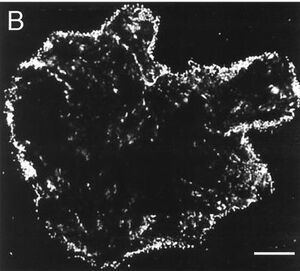
AHLs are utilized throughout members of the proteobacteria as signaling molecules for quorum-sensing based behaviors. V. paradoxus is significantly adept at utilizing these molecules as both carbon and nitrogen sources, as one publication shows that a V. paradoxus strain VAI-C was able to grow using only the AHL supplemented within media as the sole nitrogen and carbon source and this was exhibited for all AHLs tested. The ability of V. paradoxus to degrade AHLs provides strong evidence that this strain may be useful in engineering the rhizosphere to encourage/inhibit certain quorum sensing events (11). If AHLs are degraded and unable to accumulate, then certain quorum sensing behaviors will be prevented. The image below displays evidence of this degradation, as cells surrounding and likely consuming a HSL are visualized.
Degradation of Contaminants
V. paradoxus can degrade various toxins and environmental contaminants that presents this organism as a positive choice for efforts to clean the environment of toxic chemicals. One such substance that V. paradoxus aids in degradation of herbicide atrazine. Atrazine is the second most common ground and surface water contaminant within the United States, and can often be found at levels above the EPA’s recommended limit of 3ppm (parts per million) within water supplies (21). Atrazine can be metabolized both abiotic and biotically, with biotic catabolism of importance for applications for environmental clean-up. Degradation of atrazine in the environment normally requires the synergism of various bacterial species that contain one of the three genes essential for atrazine degradation. V. paradoxus contains one of these essential genes for environmental degradation of atrazine; trzD. This gene encodes a cyanuric acid amidohydrolase, which cleaves the intermediate triazine ring of atrazine metabolism. V. paradoxus is also able to aid in metabolism of simazine, a herbicide related to atrazine. In the case of simazine, V. paradoxus was also working with a consortium of other bacteria (18)
The V. paradoxus strain VM685 was found to be able to utilize 2,4-DNT as its sole nitrogen and carbon source within a nitroaromatic-contaminated site. Molecules like 2,4-DNT are used for making polyurethanes, explosives, dyes and other chemicals. In this study, V. paradoxus VM685 was found to be part of a consortium, and was responsible for the initial catalysis, in which 2,4-DNT was oxidized into 4-methyl-5-nitrocatechol (4M5NC), releasing nitrite. This was made possible by the presence of the dntAa gene, encoding 2,4-DNT dioxygenase. 4M5NC was then further oxidizied to 2-hydroxy-5-methylquinone (2H5MQ) and nitrite, which suggested DntB activity. The resulting metabolites were then metabolized by other members of the consortium (22)
V. paradoxus is capable of metabolizing Dimethyl terephthalate (DMTP), which is Used in various plastics and other polymers. This chemical is also toxic and an irritant to the skin, eyes, and respiratory tract. V. paradoxus found in marine sediments was able to degrade ambient DMTP into less harmful products. V. paradoxus has also been found to be capable of metabolizing crude oil associated metabolites like N-heterocyclic hydrocarbons (27)
Variovorax species unique metabolism also extends to bioplastic degradation, as one study isolated a Variovorax species from river water in Japan that was capable of degrading an aliphatic polycarbonate (23).
In summary, V. paradoxus is capable of promoting plant growth as well as increasing resistance to certain stressors such as drought and heavy metals. It has also shown to increase crop yield and growth when presented with these stressors. This bacterium also has a very diverse metabolism, which allows for the degradation of various chemicals, many of which are environmental contaminants. Due to the ability of V. paradoxus to metabolize such a wide array of harmful chemicals as well as its beneficial symbioses with plants and other nodule-associated rhizobia, it is a promising candidate for biotechnology applications or potential seeding of this microbe into the rhizosphere.
References
1. Arıkan, Şeyma, et al. “Physiological and Molecular Mechanisms in Improving Salinity Stress Tolerance by Beneficial Microorganisms in Plants.” Microbial Management of Plant Stresses, 2021, pp. 13–43., https://doi.org/10.1016/b978-0-323-85193-0.00006-1.
2. Belimov A. A. et al. 2005. Cadmium-tolerant plant-growth promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Siol Biol. Biochem. 37:241-250
3. Belimov et al., 2009 A.A. Belimov, I.C. Dodd, N. Hontzeas, J.C. Theobald, V.I. Safronova, W.J. Davies. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signaling New Phytol., 181 (2009), pp. 413-423, 10.1111/j.1469-8137.2008.02657.x
4. Brandt, Ulrike, et al. “Genome-Guided Insights into the Versatile Metabolic Capabilities of the Mercaptosuccinate-Utilizing β-ProteobacteriumvAriovorax ParadoxusStrain B4.” Environmental Microbiology, vol. 16, no. 11, 18 Nov. 2013, pp. 3370–3386., https://doi.org/10.1111/1462-2920.12340. Accessed 24 Apr. 2022.
5. Davies WJ, Zhang J. 1991. Root signals and the regulation of growth and development of plants in drying soil. Annual Reviews in Plant Physiology and Plant Molecular Biology 42: 55–76.
6. Dodd IC. 2005. Root-to-shoot signaling: assessing the roles of ‘up’ in the up and down world of long-distance signaling in planta. Plant and Soil 274: 251–270.
7. Greene, E A et al. “Sulfolane degradation by mixed cultures and a bacterial isolate identified as a Variovorax sp.” Archives of microbiology vol. 174,1-2 (2000): 111-9. doi:10.1007/s002030000184
8. Han, Jong-In, et al. “Complete Genome Sequence of the Metabolically Versatile Plant Growth-Promoting Endophyte Variovorax Paradoxus S110.” Journal of Bacteriology, vol. 193, no. 5, 23 Dec. 2011, pp. 1183–1190., https://doi.org/10.1128/jb.00925-10. Accessed 24 April 2022.
9. Han, Jong-In, et al. “Genome of the Root-Associated Plant Growth-Promoting Bacterium Variovorax Paradoxus Strain EPS.” Genome Announcements, vol. 1, no. 5, 31 Oct. 2013, https://doi.org/10.1128/genomea.00843-13. Accessed 24 Apr. 2022.
10. JGI. “Why Sequence Variovorax Paradoxus?” DOE Joint Genome Institute, The Regents of the University of California , 7 Nov. 2013, https://jgi.doe.gov/why-sequence-variovorax-paradoxus/.
11. Leadbetter JR, Greenberg EP. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J Bacteriol. 2000 Dec;182(24):6921-6. doi: 10.1128/JB.182.24.6921-6926.2000. PMID: 11092851; PMCID: PMC94816.
12. Maldonado, Rita F., et al. “Lipopolysaccharide Modification in Gram-Negative Bacteria during Chronic Infection.” FEMS Microbiology Reviews, vol. 40, no. 4, 12 July 2016, pp. 480–493., https://doi.org/10.1093/femsre/fuw007. Accessed 24 Apr. 2022.
13. Maimaiti J, Zhang Y, Yang J, Cen YP, Layzell DB, Peoples M, Dong Z. Isolation and characterization of hydrogen-oxidizing bacteria induced following exposure of soil to hydrogen gas and their impact on plant growth. Environ Microbiol. 2007 Feb;9(2):435-44. doi: 10.1111/j.1462-2920.2006.01155.x. PMID: 17222141.
14. Misawa, Norihiko. “Carotenoids.” Comprehensive Natural Products II, vol. 1, 2010, pp. 733–753., https://doi.org/10.1016/b978-008045382-8.00009-5. Accessed 24 Apr. 2022.
15. Ngamwonglumlert, Luxsika, and Sakamon Devahastin. “Carotenoids.” Encyclopedia of Food Chemistry, 2019, pp. 40–52., https://doi.org/10.1016/b978-0-08-100596-5.21608-9. Accessed 24 Apr. 2022.
16. Pehl, M. J., Jamieson, W. D., Kong, K., Forbester, J. L., Fredendall, R. J., Gregory, G. A., McFarland, J. E., Healy, J. M., & Orwin, P. M. (2012). Genes that influence swarming motility and biofilm formation in variovorax paradoxus EPS. PLoS ONE, 7(2). https://doi.org/10.1371/journal.pone.0031832
17. Reiter, B., and A. Sessitsch. 2006. Bacterial endophytes of the wild flower Crocus albiflorus analyzed by characterization of isolates and by a cultivation-independent approach. Can. J. Microbiol. 52:140-149.
18. Santiago-Mora, Raquel et al. “Degradation of simazine by microorganisms isolated from soils of Spanish olive fields.” Pest management science vol. 61,9 (2005): 917-21. doi:10.1002/ps.1097
19. Satola, Barbara, et al. “Metabolic Characteristics of the Species Variovorax Paradoxus.” Applied Microbiology and Biotechnology, vol. 97, no. 2, 29 Nov. 2012, pp. 541–560., https://doi.org/10.1007/s00253-012-4585-z. Accessed 24 Apr. 2022.
20. Schmalenberger, A., Hodge, S., Bryant, A., Hawkesford, M. J., Singh, B. K., & Kertesz, M. A. (2008). The role of Variovorax and other Comamonadaceae in sulfur transformations by microbial wheat rhizosphere communities exposed to different sulfur fertilization regimes. Environmental Microbiology, 10(6), 1486–1500. https://doi.org/10.1111/j.1462-2920.2007.01564.x
21. Smith, D., S. Alvey, and D. E. Crowley. 2005. Cooperative catabolic pathways within an atrazine-degrading enrichment culture isolated from soil. FEMS Microbiol. Ecol. 53:265-273]
22. Snellinx, Zita et al. “Microbial consortia that degrade 2,4-DNT by interspecies metabolism: isolation and characterisation.” Biodegradation vol. 14,1 (2003): 19-29. doi:10.1023/a:1023539104747
23. Suyama, T et al. “Bacterial isolates degrading aliphatic polycarbonates.” FEMS microbiology letters vol. 161,2 (1998): 255-61. doi:10.1111/j.1574-6968.1998.tb12956.x
24. Vogel, Jan, and Wim J. Quax. “Enzymatic Quorum Quenching in Biofilms.” Quorum Sensing, 2019, pp. 173–193., https://doi.org/10.1016/b978-0-12-814905-8.00007-1. Accessed 24 Apr. 2022
25. Wang, Yu Ping, and Ji-Dong Gu. “Degradability of dimethyl terephthalate by Variovorax paradoxus T4 and Sphingomonas yanoikuyae DOS01 isolated from deep-ocean sediments.” Ecotoxicology (London, England) vol. 15,6 (2006): 549-57. doi:10.1007/s10646-006-0093-1
26. Willems, A., et al. “Notes: Comamonadaceae, a New Family Encompassing the ACIDOVORANS Rrna Complex, Including Variovorax Paradoxus Gen. Nov., Comb. Nov., for Alcaligenes Paradoxus (Davis 1969).” International Journal of Systematic Bacteriology, vol. 41, no. 3, 1 July 1991, pp. 445–450., https://doi.org/10.1099/00207713-41-3-445. Accessed 24 Apr. 2022.
27. Willumsen, Pia Arentsen et al. “Isolation and taxonomic affiliation of N-heterocyclic aromatic hydrocarbon-transforming bacteria.” Applied microbiology and biotechnology vol. 67,3 (2005): 420-8. doi:10.1007/s00253-004-1799-8
28. Wübbeler, Jan Hendrik, et al. “The Genome of Variovorax Paradoxus Strain TBEA6 Provides New Understandings for the Catabolism of 3,3′-Thiodipropionic Acid and Hence the Production of Polythioesters.” Journal of Biotechnology, vol. 209, 10 Sept. 2015, pp. 85–95., https://doi.org/10.1016/j.jbiotec.2015.06.390. Accessed 24 Apr. 2022. .
29. UniProt ConsortiumEuropean Bioinformatics InstituteProtein Information ResourceSIB Swiss Institute of Bioinformatics. “‘Complete Genome Sequence of the Metabolically Versatile Plant Growth-Promoting Endophyte, Variovorax Paradoxus S110.".” UniProt ConsortiumEuropean Bioinformatics InstituteProtein Information ResourceSIB Swiss Institute of Bioinformatics, https://www.uniprot.org/proteomes/UP000000453.
30. “Variovorax Paradoxus S110.” BacMap, http://bacmap.wishartlab.com/organisms/896.
Author
Page authored by Sydney Szwed, Emma Seyer, and Ian Reynolds, students of Prof. Jay Lennon at Indiana University.