Vinification
Introduction
The process of making wine, also known as vinification, involves complex interactions between yeast and bacteria [1]. The rich flavours that wine is known for is the result of multiple steps of fermentation carried out in a time-dependent manner based on microbial succession of various strains of yeast and bacteria [1]. Combinations of sugar content from the fruit, temperature of storage, and fermentation time affect the proportion of products formed. Traditional methods of vinification depended on naturally occurring lactic acid bacteria (LAB) to spontaneously induce malolactic fermentation (MLF). However, with increased knowledge about the growth of microbial species in wine, MLF can now be induced at the winemaker’s discretion using starter cultures [7, 9].
Physical environment
Aged wine is a poor source of nutrients as it lacks an abundance of major bioelements including nitrogen and phosphoruous and this makes it difficult for LAB to perform MLF [1]. The low pH, high ethanol content, presence of sulphur dioxide, and low nutrition all contribute to the inhibition of yeast and bacterial growth in wine [9]. During the initial stage, wine is an aerobic environment as it is mixed to initiate alcohol fermentation in yeasts [3]. Following alcohol fermentation, the wine is completely anaerobic and LAB ferment the organic compounds produced by the yeast. However, SO2 which mainly exists as HSO3- at low pH is able to react with carbonyl compounds [3]. If the carbonyl products of the primary fermentation react with HSO3-, the organics required by LAB become unavailable for fermentation [3].
Biological interactions
Because wine is a complex system of microbial activity, there are many interactions among various yeasts and bacteria. Yeasts produce many inhibitory compounds that reduce the amount of bacterial growth, such as SO2, ethanol, medium chain fatty acids, and antimicrobial peptides [1, 3]. High levels of medium chain fatty acids and a very acidic environment can inhibit the ATPase activity of O. oeni, which can reduce the rate of MLF [9]. In addition, the completion of alcohol fermentation by yeasts yields many missing substances, like vitamins and amino acids, which allow LAB to thrive [1]. One example of a bacterial interaction that can have negative consequences on vinification is the production of Brevicin. Brevicin is a bacteriocin produced by Lactobacillus brevis that inhibits the growth of O. oeni [1].
Microbial processes
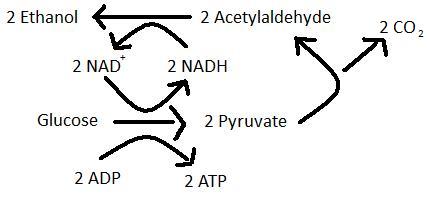
Alcohol fermentation is the primary fermentation of wine in which sugars such as glucose are converted to ethanol and carbon dioxide. The products of alcohol fermentation combine with grape juice to produce a pleasant aroma and flavour [8]. Saccharomyces cervisiae is a specialized yeast species that will preferentially ferment in both aerobic and aneraobic conditions [10].
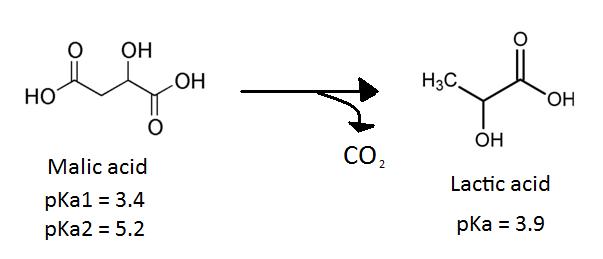
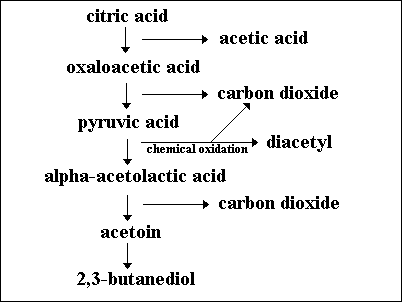
The secondary fermentation of wine, also known as MLF, is the enzymatic decarboxylation of L-malic acid to L-lactic acid [3]. MLF leads to the deacidifcation and stabilization of wine and results in an increase in growth rate of LAB as well as an enhancement of the wine’s flavour and aroma [9]. The wine’s flavour is enhanced during MLF because LAB produce aromatic compounds by hydrolyzing glycosides of the aroma compounds in the grape juice [11]. Certain ester compounds that are formed by a reaction between organic acids can give wine an aroma of vanilla or strawberries [1].
Fermentation of the citric acid that is also present in the grape juice by LAB results in the formation of diacetyl, a compound that gives wine a buttery or nutty flavour [3]. However, acetic acid and ethyl acetate are fermentation products that need to be avoided because they give wine an undesirable flavour [5]. The metabolism of each microorganism is a large determinant in the final taste and smell of wine.
Key Microorganisms
Saccharomyces cervisiae
Saccharomyces is a genus of unicellular fungi that can grow in both aerobic and anaerobic environments where they preferentially ferment, rather than respire, in the presence of a fermentable sugar. Saccharomyces cervisiae is the main contributor of alcohol fermentation because of its tolerance to high concentrations of ethanol [10]. Pina et al. (2004) determined that S. cervisiae is able to modify the lipid composition of its plasma membrane according to the increasing stress applied by the increasing ethanol concentration [10].
Lactobacillus plantarum
Lactobacillus plantarum is a species of Gram-positive microaerophilic bacteria that require rich media with a fermentable sugar to grow [1]. Because these organisms are specialists in lactic acid fermentation, they lack the ability to respire and are obligately fermentative with a limited substrate range and limited biosynthesis [13]. L. platarum are homofermentative as they only produce lactic acid as an end product and are involved in the secondary fermentation of wine by contributing to MLF [1].
Oenococcus oeni
Oenococcus oeni is a species of Gram-positive acidophilic facultative anaerobes that can grow at a pH of 4.8 at 18-30 degrees Celsius. They require rich media with tomato or grape juice to grow and are heterofermentative organisms that can produce products other than lactic acid [1]. However, O. oeni is ideal for the secondary fermentation of wine because it preferentially ferments malic acid to lactic acid and does not produce many waste products that will negatively impact the flavour of wine [1, 2]. O. oeni can be inoculated into wine to control the timing and rate of MLF and reduce the chance of spoilage by other bacterial species [3].
These organisms are the main contributors of making wine because they are not inhibited by the high level of ethanol produced after alcohol fermentation is complete [1, 3]. O. oeni and other LAB rely on ATPase activity to maintain their intracellular pH while growing in acidic environments [6]. In addition, S. cervisiae, O. oeni, and L. platarum contain a lactate/proton symport that uses the lactate concentration gradient to drive proton translocation out of the cell [12, 13].
Current Research
After analysis of LAB in vinification, it was discovered that LAB are able to inhibit the growth of pathogenic bacteria through their ability to outcompete pathogens for nutrients, survive the low pH, and secrete antimicrobial compounds [4]. It was previously thought that by inoculating O. oeni into raw food, food-borne pathogens including Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella enteridis can be controlled [4]. Chiang et al. (2012) tested the ability of O. oeni to inhibit the growth of these common pathogens and found that of the 24 strains of O. oeni tested, 17 strains were able to inhibit E. coli O157:H7, L. monocytogenes, and S. enteridis [4]. The application of O. oeni in pathogen control will have major economical, ecological, and health implications, but the safety of such a method needs to be confirmed by further testing.
References
(1) Costantini, A., Garcia-Moruno, E., and Moreno-Arribas, M.V. “Biochemical Transformations Produced by Malolactic Fermentation.” Wine Chemistry and Biochemistry, 2009, doi: 10.1007/978-0-387-74118-5 2
(2) Borneman, A.R., McCarthy, J.M., Chambers, P.J., and Bartowsky, E.J. “ Comparative analysis of the Oenococcus oeni pan genome reveals genetic diversity in industrially-relevant pathways.” BMC Genomics, 2012, doi: 10.1186/1471-2164-13-373
(3) Nielsen, J.C., and Richelieu, M. “Control of Flavor Development in Wine during and after Malolactic Fermentation by Oenococcus oeni.” Applied and Environmental Microbiology, 1999, 0099-2240/99
(4) Chiang, I., Worobo, R.W., Churey, J.J., and Henick-Kling, T. “Growth Inhibition of Foodborne Pathogens by Oenococcus oeni.” Journal of Food Science, 2011, doi: 10.1111/j.1750-3841.2011.02446.x
(5) Campos, F.M., Couto, J.A., and Hogg, T.A. “Influence of phenolic acids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii.” Journal of Applied Microbiology, 2003.
(6) Carrete, R., Vidal, M.T., Bordons, A., and Constanti, M. “Inhibitory effect of sulfur dioxide and other stress compounds in wine on the ATPase activity of Oenococcus oeni.” FEMS Microbiology Letters, 2002, doi: 0378-1097 / 02
(7) Mangani, S. Guerrini, S., Granchi, L., and Vincenzini M. “Putrescine Accumulation in Wine: Role of Oenococcus oeni.” Current Microbiology, 2005, doi: 10.1007/s00284-004-4425-1
(8) D’Incecco, N., Bartowsky, E., Kassara, S., Lante, A., Spettoli, P., and Henschke, P. “Release of glycosidically bound flavour compounds of Chardonnay by Oenococcus oeni during malolactic fermentation.” Food Microbiology, 2004, doi: 10.1016/j.fm.2003.09.003
(9) Alexandre, H., Costello, P.J., Remize, F., Guzzo, J., and Guilloux-Benatier, M. “Saccharomyces cerevisiae–Oenococcus oeni interactions in wine: current knowledge and perspectives.” International Journal of Food Microbiology, 2004, doi: 10.1016/j.ijfoodmicro.2003.10.013
(10) Pina, C., Santos, C., Couto, J.A., and Hogg, T. “Ethanol tolerance of five non Saccharomyces wine yeasts in comparison with a strain of Saccharomyces cerevisiae—influence of different culture conditions.” Food Microbiology, 2004, doi: 10.1016/j.fm.2003.10.009
(11) Ugliano, M., Genovese, A., and Moio, L. “Hydrolysis of wine aroma precursors during malolactic fermentation with four commercial starter cultures of Oenococcus oeni.” Journal of Agricultural and Food Chemistry, 2003, doi: 10.1021/jf0342019
(12) Cassio, F., Leao, C., and van Uden, N. “Transport of lactate and other short-chain monocarboxylates in the yeast Saccharomyces cerevisiae.” Applied and Environmental Microbiology, 1987.
(13) Goncalves, L.M.D., Ramos, A., Almeida, J.S., Xavier, A.M.R.B., and Carrondo M.J.T. “Elucidation of the mechanism of lactic acid growth inhibition and production in batch cultures of Lactobacillus rhamnosus.” Applied Microbiology and Biotechnology, 1997.