Zooxanthellae and their Symbiotic Relationship with Marine Corals
by Charlotte Leblang
Introduction
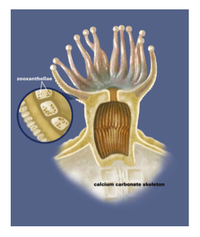
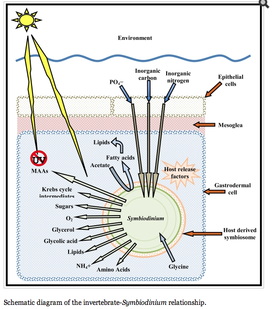
Zooxanthellae is the brown-yellow algae that lives in coral’s gastrodermis, and is the common name of the broader Symbiodinium genus (3). Zooxanthellae is a term for any dinoflagellate that participates in symbiosis with sponges, coral, clams, mollusks, flatworms, jellyfish, etc (1,2). It is an algal protist that is best known for its symbiotic relationship with marine coral. Zooxanthellae usually occur in extremely high densities on their host, enhancing the constant exchange of nutrients between them and their host (Figure 1).
Corals are usually colonies of polyps. Polyps are live coral tissue extensions that cover the calcium carbonate structure, and are usually only a few millimeters thick. The tissue has two layers, the epidermis and the gastrodermis, where the zooxanthellae live (36). Zooxanthellae are unicellular and spherical with two flagella that fall off once they are incorporated within a host. Zooxanthellae undergo asexual reproduction by division, and most of their energy comes from performing photosynthesis using the byproducts of cellular respiration produced from the host coral.
Life Cycle
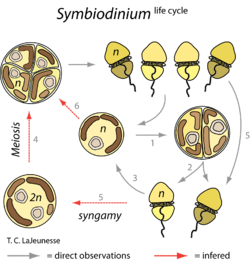
Their life cycle is in two stages: the coccoid stage and the motile masticate phase (Figure 2). In the motile phase, the zooxanthellae retain their flagella and are free-living. In the coccoid stage, the stage in which zooxanthellae are more commonly found, the zooxanthellae are intracellular symbionts within the coral and do not keep their flagella. During reproduction, the chromosomal and nuclear division occurs in darkness, while the cellular division into two flagellated cells (cytokinesis) occurs in exposure to light. This division in log phase is about every one to three days, but in culture division slows during stationary phase and fewer motile cells are produced. Mitosis occurs on the coccoid cells as well, which are surrounded by a cell wall of glycoproteins and proteins, and only one species of zooxanthellae is known to have surface projections (13). The zooxanthellae’s chloroplast has three membranes, and the thylakoid membranes differ between species.
Genome
The Symbiodinium genome was very recently sequenced. It was found that the genome contains unidirectionally aligned genes and that these genes form a cluster-like arrangement. There is an estimated 1,500 Mbp in the genome of the species Symbiodinium minutum and approximately 42,000 protein-encoding genes. There are also genes to regulate chromosome condensation proteins, and about two-thirds of these genes were obtained through bacterial horizontal transfer, while the other one-third most likely have eukaryotic orthologs. There are unique donor and acceptor splice sites (4).
Coral Bleaching
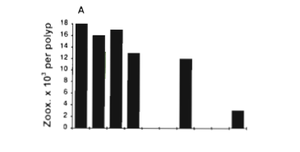
What exactly is coral bleaching? Well the zooxanthellae are prominent on the coral; there are approximately 1-5*10^6 zooxanthellae per cm^2 and each zooxanthella has about 2-10pg of chlorophyll. When coral bleaching occurs, the coral lose about 60-70% of their zooxanthellae, which in turn lose 50-80% of their photosynthetic pigments (5). The coral becomes bleached because it expels the zooxanthellae, leaving a bare skeleton of calcium carbonate because the algae is what gave the coral its color. The most common reasoning behind why the zooxanthellae leaves the coral is the idea that sudden high water temperature or uncomfortable environmental conditions will expel the algae in the open water (Figure 3). When the algae leaves the coral, the coral begins to starve, but if the optimal conditions return soon there is hope that the zooxanthellae will come back. If the algae do not come back because the stress is still present, however, then the coral will die. In a paper discussing the effects Hurricane Flora had on coral reefs in Jamaica, it was found that some zooxanthellae did in fact reinhabit the coral after some time, thus making part of the reef salvageable after the natural disaster (37). Interestingly, however, it was found that perhaps the differentiation of lipids in the Symbiodinium could cause varying sensitivity to thermal stress. In one study it was found that more disorganized stacking in the thylakoid membrane resulted from the Symbiodinium being exposed to high temperatures. This showed that the composition of the lipids might be important to understanding the temperature range of the algae (24).
Besides the direct loss of zooxanthellae, coral bleaching can occur in other ways. UV and visible light have both been shown to have a role in coral bleaching, along with subaerial exposure, which causes an inconsistent environment for the coral. Furthermore, sedimentation has been thought to induce coral bleaching, along with dilution of waters or an influx of inorganic ingredients into the ecosystem. Also, pollution and pathogens are understandably a cause for coral bleaching to occur (5).
Some of the symbiotic organisms do have a defense against the UV light, however. Mycosporine-like amino acids (MAAs) can uptake the UV light and do not require extra reactions to do so. The MAAs can also uptake radicals, but are not found in every clade of Symbiodinium (29). A study in 2000 showed that two of the three clades observed did not produce these MAAs, and the one clade that did had an increase of them during the middle of the day. This implies that some species of the Symbiodinium have adapted to the UV radiation, while some still have not, and perhaps in the future the algae with the ability to survive will attach to the majority of the coral so UV radiation will no longer be a threat to reefs.
Global Warming
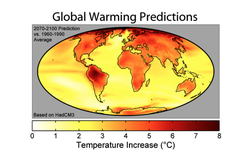
Due to the implication that an increase in ocean water temperature will bleach coral, global warming becomes an increasingly hot topic (Figure 4). It is expected that if the ocean warms just one to two degrees, the locations that are between twenty and thirty degrees North will then fall within the range of lethality for most coral species. Some may be able to adapt, but typically the photosynthesis pathways are hindered at temperatures rising above thirty degrees Celsius. Thus, temperature shocks resulting from global warming results in zooxanthellae adhesion dysfunction, so they detach and are expelled from the coral (5). In a study from 2012, it was shown that the Symbiodinium density significantly decreased after twenty-seven days of heat stress (11). Furthermore, another study looked directly at photosystem genes in response to thermal stress, and both had significant declines when exposed to 32˚C over a period of time (34).
Diversity
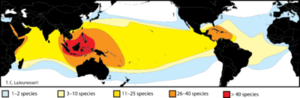
Some coral species can increase their tolerance to temperature changes by zooxanthellae clade shuffling. In other words, different zooxanthellae are sensitive to different temperatures, and coral can expel the old algae in hopes that the less sensitive algae will have survived and become a new symbiont. This is an idea among scientists because zooxanthellae species diversity is very widely spread (Figure 5). Horizontal gene transfer and many genetic lineages make up the Symbiodinium species, causing disparity among the clades. So although there are many Symbiodinium-like species, this idea of clade shuffling seems slightly implausible, because it usually is a matter of 1-1.5 degrees of temperature fluctuation (8). Another study focused on the classification of zooxanthellae (31). They isolated compounds that were later identified as toxins that were unique from other dinoflagellates. The discovery and research into these compounds also supported that the molecules were from the algae and not a result of the host, but it seemed that variation to the host and environment caused the production of different algal metabolites. Many other toxins and compounds were isolated in this study and added significantly to the fact that the metabolism and taxon of zooxanthellae are extremely diverse. Furthermore, it has been shown that specific Symbiodinium are more tolerant to heat and stress, and perhaps corals adopting these specific algae will be able to survive the temperature changes from global warming and natural disasters (32). Another study found that following bleaching, corals had clade shuffled from C2 to D, because D has a higher densities and photochemical efficiency, resulting in higher thermal tolerance (33).
Symbiosis
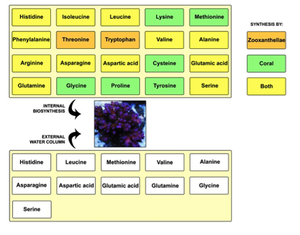
The symbiotic relationship between zooxanthellae and marine coral is understood on a basic level. The coral polyps do cellular respiration, thus producing carbon dioxide and water as byproducts. The zooxanthellae then take up these byproducts to carry out photosynthesis. The products of photosynthesis include sugars, lipids, and oxygen, which the coral polyps thus uptake for growth and cellular respiration, and the cycle continues. The photosynthesis byproducts are more specifically used to make proteins and carbohydrates in order to produce calcium carbonate for the coral to grow. Furthermore, the oxygen is used by the coral to help remove wastes. This recycling of nutrients in between these symbionts is extremely efficient, resulting in the ability to live in nutrient poor waters. About ninety percent of the material produced by photosynthesis is thought to be used by the coral (6). In terms of disease, the zooxanthellae is commonly the point of attack, rather than the coral itself. For example, the Montastrae species, which causes Yellow Band Disease, affects the zooxanthellae directly rather than the coral (7).
Besides the nutrient shuffling, there seems to be another level to the zooxanthellae-coral symbiotic relationship. Scientists found that a coral, Acropora, lacked an enzyme needed for cysteine biosynthesis. It thus needed Symbiodinium for the production of this amino acid. The genome size for the zooxanthellae algae is about 1,500 Mbp while the coral is approximately 420 Mbp: the coral most likely rely on the algae for more than just the enzyme needed for cysteine biosynthesis (9). Sure enough, other studies have shown phosphate-linked relationships between these two species. Zooxanthellae extracted from the Acropora coral had two acid phosphatases P-1 and P-2. The activity of these enzymes shows that perhaps their role is involved in the mobilization of a phosphate storage compound. The exact role of these enzymes is unknown, but it seems that the symbiotic relationship between coral and zooxanthellae is phosphate limited (10). Another study found that in this coral transcriptome study, approximately 35% of sequences originated in the symbiont, but are essential to both the coral and Symbiodinium (16). But together, the coral and zooxanthellae can synthesize twenty amino acids (17) (Figure 6).
There is also a relationship between the amount of time the tentacles of the coral spend expanded or contracted and the amount of zooxanthellae present on the coral. In general, there was lower photosynthetic efficiency in the zooxanthellae coral species that has their tentacles expanded only at night than the species with their tentacles constantly expanded. Also, the zooxanthellae density was higher in the continuously expanded tentacle species. These differences were found only in the light however, because when the species were placed in the dark no differences were found. Thus the light has a relationship with the coral and zooxanthellae, which was assumed because zooxanthellae are photosynthetic organisms. Conclusively, the species with continuously expanded tentacles have dense populations or small tentacles. The findings suggest that small tentacles do not shade the zooxanthellae, thus they are all visible to the light, and that dense populations are necessary to harvest the light. So the species with these proactive properties expand continuously to collect all the light, while the species with few zooxanthellae only expand at night (12). Another study related the exposure of the coral to oxygen as a means for oxygen radical accumulation in its tissues (28). The O2 concentrations were found to increase by a pH of about 1.2 just by moving from light to dark, and the concentrations rose about 250%. Thus causes an increase of oxygen radicals in the coral tissues from the molecular oxygen, and the radicals can destroy cells. This study found that the anemones with higher chlorophyll, and thus higher Symbiodinium, actually adjusted their protein expression so the fluctuating oxygen concentrations would not be destructive. This is just another example of how the coral changes its innate reactions to adjust for its symbiotic algae (Figure 7).
Movement
Furthermore, it was found that the temperate symbiotic sea anemone, Anthropluera balli, incorporates a maternal inheritance of the zooxanthellae because the anemone live in locations of low zooxanthellae algae. It was found that the spawned ova consistently contained zooxanthellae, and were released into the ocean water to become fertilized and grow. The zooxanthellae was clearly integrated into the life cycle of this particular sea anemone, and was found to localize at one end of the embryo to become integrated within the endoderm, which as mentioned above is where the zooxanthellae live within coral (14). This study brings arise the question of how zooxanthellae disperse among the coral. Another study discovered that the zooxanthellae can be released by the host in ways such as predation, extrusion, spontaneously, osmotically, or as we know, due to temperature or stress. This particular study proposes another way for zooxanthellae to disperse, through the feces of their predators. Interestingly, photosynthetic rates from the unharmed species were very similar to the rates from the fecal zooxanthellae that made their way through a digestive tract. Furthermore, the zooxanthellae reinfected sea anemones after their travel through the digestive tract of their predator. This finding showed that predation is an important means by which the zooxanthellae are dispersed among a coral reef (15).
History
The relationship between Symbiodinium and coral has been known for about fifty years. One of the first studies found that certain dinoflagellates fixed labeled carbon from CO2 and moved it to their host sea anemone after forty-eight hours. This study also showed that Symbiodinium produced higher amounts of carbohydrates when living inside a host rather than free living (18). After this symbiotic relationship was discovered, other studies delved further into how the algae and coral used the nutrients they acquired from the other. One study found specifically that the algae fixed the carbon primarily as glycerol, which was then taken up by the coral tissue as proteins and lipids (19). It was also discovered that the other organic acids produced by the Symbiodinium were different biochemically, even though they looked the same (20). This information was the beginning of other scientists discovering the increasingly wide variety in the taxon of dinoflagellates. This same study also discovered that the compounds produced by the algae were different when cultured than when in ocean water, thus indicating that perhaps the coral symbiont has a role in regulating the algae’s metabolite production or biosynthesis. It is not entirely sure how the coral does this, but some studies have hypothesized. Grant et. al. have proposed that the coral synthesize a peptide that is extremely low in molecular weight, and that is able to impair the photosynthesis of Symbiodinium (21). Other studies suggest that the host coral produces compounds that act as host release factors, and that these factors can control the metabolite production in the Symbiodinium (22).
Energy Storage
Not only are nutrients shared between the two species, but energy and energy production is integrated as well. Patton et al. showed that the energy reserved by the host coral, in the form of lipids, was produced by the Symbiodinium but stored in the coral’s tissues. The Symbiodinium produced these lipids, using acetate from the coral and extra ATP, and excreted them back to their host. The Symbiodinium was found to have 8% of these lipids for themselves, while 75% were transferred back to the host. These lipids are mostly wax esters and triglycerides (23).
Ammonium
Another molecule that is transferred between the algae and the host coral is ammonium. A study showed that the corals’ uptake of ammonium was positively correlated with light (this relates back to the idea of tentacles constantly expanding or expanding only at night) (25). It was further shown that the retention of this ammonium by the coral was related to the Symbiodinium because the algae uptakes most of the ammonium itself (26). The algae were also more efficient with its use of a nitrogen source because it can use nitrite. A study used tagged enzymes involved in the use of different forms of nitrogen, and concluded that the algae do indeed utilize nitrates. They also found that the algae densities increase with the nitrate concentration, although further details of this relationship with the coral are not known (27). It is also interesting to note that the MAA concentration, which usually increases with UV exposure, also increased at high ammonium concentrations (30). This study was done in red algae, Porphyra, but still may provide information regarding the zooxanthellae and its symbiotic relationship with corals (Figure 8).
Human Threat

Humans are also directly involved in the loss of coral; over ten percent of coral reefs have been destroyed (35). Some fishing practices involve blowing up reefs with explosives to stun the fish so the fisherman can catch them easily (Figure 9). This completely destroys the coral reefs and the habitat—the fish that are left don’t have a place to live anymore. Another fishing practice that is particularly detrimental is fishing with cyanide. Divers pour cyanide, a poison, on the reefs to stun the fish. This poison kills the coral and makes the fish that aren’t caught extremely sick until they also die. The divers also directly rip coral off the reef to catch the hiding and sick fish. These practices of fishing are completely destroying the reefs and environment. Also, as we saw above, some fish that are predators of the zooxanthellae actually disperse the algae in their feces. Due to overfishing, this dispersion technique may no longer be available, thus diminishing the diversity of zooxanthellae, and therefore coral, around the oceans. Also, coral is very delicate, and divers merely touching the coral can damage years of growth. It is also thought that the oils from a human can be harmful towards the coral and algae living within or on it; tourism perhaps has been degrading coral for years.
Conclusion
Zooxanthellae and coral have clearly been shown to have a close-knit symbiotic relationship. The most prominent research topic is the discussion regarding coral bleaching. The zooxanthellae are expelled from the coral in stress situations, most recently due to the rising ocean water temperatures. The enzyme, nutrient, and molecule cycling between the algae and the coral are extremely co-dependent, and the loss the algae clearly results in coral bleaching and death. The organisms protect each other, whether from UV radiation or predation, although it seems humans can surpass all natural protection and destroy the coral by merely overfishing or stepping on it. The loss of the coral has a large global impact because it is a home for a large number of fish and other marine creatures. We are learning that it is necessary to be aware of not only the coral itself, but of the organisms that live in the reefs or within the coral.
References
1. "Reefs.org: Where Reefkeeping Begins on the Internet." Where Reefkeeping Begins on the Internet. N.p., n.d. Web. 23 Apr. 2014. <http://www.reefs.org/library/talklog/l_ho_030898.html>.
2. "Zooxanthellae." - MicrobeWiki. N.p., n.d. Web. 23 Apr. 2014. <https://microbewiki.kenyon.edu/index.php/Zooxanthellae>.
3. "Symbiotic Algae." NOAA's Coral Reef Conservation Program:. N.p., n.d. Web. 23 Apr. 2014. <http://coralreef.noaa.gov/aboutcorals/coral101/symbioticalgae/>.
4. Shoguchi, Eiichi, et. al. "Draft Assembly of the Symbiodinium Minutum Nuclear Genome Reveals Dinoflagellate Gene Structure." Current Biology 23.15 (2013): 1399-408. Print.
5. Buchheim, Jason. "Coral Reef Bleaching." Coral Reef Bleaching. N.p., n.d. Web. 23 Apr. 2014. <http://www.marinebiology.org/coralbleaching.htm>.
6. "NOAA's National Ocean Service: Diagram of Coral and Zooxanthellae Relationship." NOAA's National Ocean Service: Diagram of Coral and Zooxanthellae Relationship. N.p., n.d. Web. 23 Apr. 2014. <http://oceanservice.noaa.gov/education/kits/corals/media/supp_coral02bc.html>.
7. "Zooxanthellae." - MicrobeWiki. N.p., n.d. Web. 23 Apr. 2014. <https://microbewiki.kenyon.edu/index.php/Zooxanthellae>.
8. Adaptations of Corals and Coral Reefs to Climate Change. Digital image. N.p., n.d. Web. 23 Apr. 2014. <http://www.conference.ifas.ufl.edu/ncer2009/PPTPDF_pres/4-Thursday/1-San%20Jose/PM/0320%20S%20Colley.pdf>.
9. Okinawa Institute of Science and Technology - OIST. "A coral symbiont genome decoded for first time." ScienceDaily. ScienceDaily, 12 July 2013. <www.sciencedaily.com/releases/2013/07/130712084329.htm>.
10. Jackson, A. E., et. al. "Phosphorus Metabolism in the Coral-Zooxanthellae Symbiosis: Characterization and Possible Roles of Two Acid Phosphatases in the Algal Symbiont Symbiodinium Sp." Proceedings of the Royal Society B: Biological Sciences 238.1291 (1989): 193-202. Print.
11. Bellantuono, Anthony J., et. al. "Coral Thermal Tolerance: Tuning Gene Expression to Resist Thermal Stress." PLoS ONE 7.11 (2012): E50685. Print.
12. Levy, O. "Photobehavior of Stony Corals: Responses to Light Spectra and Intensity." Journal of Experimental Biology 206.22 (2003): 4041-049. Print.
13. "Symbiodinium." Wikipedia. Wikimedia Foundation, 21 Apr. 2014. Web. 23 Apr. 2014. <http://en.wikipedia.org/wiki/Symbiodinium>.
14. Davy, Simon K., and John R. Turner. "Early Development and Acquisition of Zooxanthellae in the Temperate Symbiotic Sea Anemone Anthopleura Ballii (Cocks)." Biological Bulletin 205 (2003): 66-72. Web.
15. Parker, Gisele M. "DISPERSAL OF ZOOXANTHELLAE ON CORAL REEFS BY PREDATORS ON CNIDARIANS." Biological Bulletin 167 (1984): 159-67. Web.
16. Shinzato, Chuya., et. al. "A Snapshot of a Coral “Holobiont”: A Transcriptome Assembly of the Scleractinian Coral, Porites, Captures a Wide Variety of Genes from Both the Host and Symbiotic Zooxanthellae." PLoS ONE 9.1 (2014): E85182. Print.
17Wijgerde, Tim. "Aquarium Corals: Amino Acids and Corals: Sources, Roles and Supplementation." — Advanced Aquarist. N.p., n.d. Web. 23 Apr. 2014. <http://www.advancedaquarist.com/2014/3/corals>.
18. Muscatine, L. "Direct Evidence for the Transfer of Materials from Symbiotic Algae to the Tissues of a Coelenterate." Proceedings of the National Academy of Sciences 44.12 (1958): 1259-263. Print.
19. Trench RK. The Physiology and Biochemistry of Zooxanthellae Symbiotic with Marine Coelenterates. I. The Assimilation of Photosynthetic Products of Zooxanthellae by Two Marine Coelenterates. Proc. R. Soc. Lond. B Biol. Sci. 1971;177:225–235.
20. Trench RK. The Physiology and Biochemistry of Zooxanthellae Symbiotic with Marine Coelenterates. II. Liberation of Fixed 14C by Zooxanthellae in Vitro. Proc. R. Soc. Lond. B Biol. Sci. 1971;177:237–250.
21. Grant AJ, Remond M, Withers KJT, Hinde R. Inhibition of Algal Photosynthesis by a Symbiotic Coral. Hydrobiologia. 2001;461:63–69.
22. Grant AJ, Remond M, Hinde R. Low Molecular-Weight Factor from Plesiastrea versipora (Scleractinia) That Modifies Release and Glycerol Metabolism of Isolated Symbiotic Algae. Mar. Biol. 1998;130:553–557.
23. Patton JS, Abraham S, Benson AA. Lipogenesis in the Intact Coral Pocillopora capitata and Its Isolated Zooxanthellae: Evidence for a Light-Driven Carbon Cycle between Symbiont and Host. Mar. Biol. 1977;44:235–247.
24. Tchernov D., et.al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci U S A. 2004 Sep 14; 101(37):13531-5.
25. Muscatine L, D’Elia CF. The Uptake, Retention, and Release of Ammonium by Reef Corals. Limnol. Oceanogr. 1978;23:725–734.
26. D’Elia CF, Domotor SL, Webb KL. Nutrient Uptake Kinetics of Freshly Isolated Zooxanthellae. Mar. Biol. 1983;75:157–167.
27. Marubini F, Davies PS. Nitrate Increases Zooxanthellae Population Density and Reduces Skeletogenesis in Corals. Mar. Biol. 1996;127:319–328.
28. Kuhl M, Cohen Y, Dalsgaard T, Jorgensen BB, Revsbech NP. Microenvironment and Photosynthesis of Zooxanthellae in Scleractinian Corals Studies with Microsensors for O2, pH and Light. Mar. Ecol. Prog. Ser. 1995;117:159–172.
29. The synthesis of mycosporine-like amino acids (MAAs) by cultured, symbiotic dinoflagellates.T Banaszak., et. al. J Exp Mar Bio Ecol. 2000 Jun 28; 249(2):219-233.
30. Korbee N, Houvinen P, Figueroa FL, Aguilera J, Karsten U. Availability of Ammonium Influences Photosynthesis and the Accumulation of Mycosporine-Like Amino Acids in Two Porphyra Species (Bangiales, Rhodophyta) Mar. Biol. 2005;146:645–654.
31. Isolation of zooxanthellatoxins, novel vasoconstrictive substances from the zooxanthella Symbiodinium sp. Nakamura H, Asari T, Ohizumi Y, Kobayashi J, Yamasu T, Murai A Toxicon. 1993 Apr; 31(4):371-6.
32. Abrego, David., et. al. "Species–specific Interactions between Algal Endosymbionts and Coral Hosts Define Their Bleaching Response to Heat and Light Stress." Proceedings of the Royal Society B: Biological Sciences 275.1648 (2008): 2273-282. Print.
33. The role of zooxanthellae in the thermal tolerance of corals: a 'nugget of hope' for coral reefs in an era of climate change.Berkelmans R, van Oppen MJProc Biol Sci. 2006 Sep 22; 273(1599):2305-12.
34. Mcginley, Michael P., et. al. "Transcriptional Response of Two Core Photosystem Genes in Symbiodinium Spp. Exposed to Thermal Stress." PLoS ONE 7.12 (2012): E50439. Print.
35. "CORAL REEF DESTRUCTION AND CONSERVATION - Coral Reefs - Ocean World." CORAL REEF DESTRUCTION AND CONSERVATION - Coral Reefs - Ocean World. N.p., n.d. Web. 21 Apr. 2014. <http://oceanworld.tamu.edu/students/coral/coral5.htm>.
36. Parker, Gisele M., and Christopher F. D'Elia. "Interactions between Corals and Their Symbiotic Algae." (n.d.): n. pag. Web. <http://www.marine.usf.edu/reefslab/documents/evol_ecol2007/Muller-Parker%26DeliaCh5_rev.pdf>.
37. Goreau, T. F. "Mass Expulsion of Zooxanthellae from Jamaican Reef Communities after Hurricane Flora." Science 145.3630 (1964): 383-86. Print.