Fungal Endophytes: Drought Tolerance in Plants: Difference between revisions
SBarnes7151 (talk | contribs) No edit summary |
SBarnes7151 (talk | contribs) No edit summary |
||
| (35 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
<br>It has been estimated that over 80% of terrestrial plants form a symbiotic association with fungi.[[#References |[1]]] | <br>It has been estimated that over 80% of terrestrial plants form a [http://en.wikipedia.org/wiki/Symbiosis symbiotic] association with fungi.[[#References |[1]]] Species of fungi that reside within living plant tissue without causing symptoms of disease in their host are known as fungal [https://microbewiki.kenyon.edu/index.php/Plant_endophyte endophytes].[[#References |[4]]] Fungal endophytes colonize a variety of both [http://en.wikipedia.org/wiki/Monocotyledon monocot] and [http://en.wikipedia.org/wiki/Eudicots eudicot] plants which suggests that this symbiosis predated the monocot-dicot split 140-150 million years ago.[[#References |[2]]] It is also hypothesized that phototroph-fungi associations enabled plants to first colonize land.[[#References |[3]]] This mutualistic association could have helped plants acclimate to new environmental stresses such as desiccation, increased exposure to solar radiation, and more extreme temperatures differences.[[#References |[3]]] | ||
<br><br>Fungal endophytes remain an important component of today’s terrestrial ecosystems, and many enable their hosts to thrive in harsh environments. Studies show that fungal endophytes can enhance the drought, salt, and soil temperature tolerance of their host plant in addition to increasing resistance to parasitic fungi and herbivores.[[#References |[4]]] With growing concerns about climate change and its effects on agriculture, learning about fungal endophyte conferred drought tolerance has become increasingly important. By influencing plant morphology, development, and physiological and biochemical responses to stress, fungal endophytes can induce mechanisms of drought avoidance, [http://en.wikipedia.org/wiki/Drought_tolerance drought tolerance], and drought recovery in their hosts.[[#References |[8]]] | |||
<br><br>Studies | |||
==Classes of Fungal Endophytes== | ==Classes of Fungal Endophytes== | ||
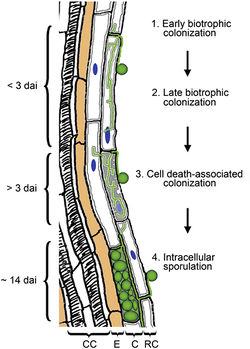
===Clavicipitaceous Endophytes | [[File:Jacobs_et_al_(2011)_crop.jpeg|thumb|250px|right| [http://www.plantphysiol.org/content/156/2/726/F10.expansion.html <b> Figure 1. ]Four stages of root colonization by Class 2 endophyte <i> Piriformospora indica </i>. </b> <b>1.</b> Extracellular colonization of the root surface. <b> 2. </b> The [http://en.wikipedia.org/wiki/Hypha hyphae] penetrate the epidermal (RC) and [http://en.wikipedia.org/wiki/Cortex_(botany) cortical] (C) cells. Cell organelles (e.g. nucleus; blue) remain intact and plasma membrane invaginate (dark gray lines inside cells). <b> 3. </b> The plasma membrane still surrounds intracellular hyphae while biotrophically colonized cells die (light gray filling of cells) and organelles are disrupted. <b> 4. </b> Fungal reproduction in RC and C cells. Endodermis cells (E) are not colonized.[[#References |[13]]] ]] | ||
===Clavicipitaceous Endophytes=== | |||
====Class 1==== | ====Class 1==== | ||
Clavicipitaceous endophytes are [https://microbewiki.kenyon.edu/index.php/Grasses_and_endophytic_fungi associated with grasses].[[#References |[4]]] They are typically found within the plant shoots and form systemic intercellular infections.[[#References |[4]]] While they are often passed down in the seed through [http://en.wikipedia.org/wiki/Vertically_transmitted_infection vertical transmission], they may also undergo [http://en.wikipedia.org/wiki/Horizontal_disease_transmission horizontal transmission].[[#References |[4]]] Many Class 1 endophytes produce [http://en.wikipedia.org/wiki/Alkaloid alkaloids] to protect their host plant from herbivory by insects and mammals, and studies have shown some Class 1 endophytes to confer drought and metal tolerance.[[#References |[5]]][[#References |[8]]] <i>[https://microbewiki.kenyon.edu/index.php/Neotyphodium_coenophialum Neotyphodium coenophialum] </i>, for example, stimulates plants to develop more extensive root systems and longer and thinner root hairs which increases root surface area for improved water and mineral acquisition. [[#References |[8]]] | |||
===Nonclavicipitaceous Endophytes=== | |||
Nonclavicipitaceous endophytes are highly diverse and have been isolated from every major lineage of land plant, which includes nonvascular plants, ferns, conifers, and angiosperms. | |||
====Class 2==== | ====Class 2==== | ||
Class 2 endophytes are usually found in the roots, stem, or leaves of their hosts.[[#References |[4]]] Like Class 1 endophytes, they can also be transmitted either vertically through the seed coat or horizontally. [[#References |[4]]] They are unique in that they can confer habitat-specific stress tolerance to their hosts, and, as a result, they infect a higher percentage of plants in high-stress environments.[[#References |[4]]] They often increase root and/or shoot biomass in their host.[[#References |[4]]] | |||
====Class 3 and 4==== | ====Class 3 and 4==== | ||
Class 3 fungal endophytes colonize the shoot of plants while Class 4 endophytes colonize plant roots.[[#References |[4]]] Both are horizontally transmitted.[[#References |[4]]] Unlike the other classes, Class 3 endophyte colonizations are highly localized, and a diverse number of species can colonize an individual plant.[[#References |[4]]] Few studies have been performed on Class 3 and 4 endophytes and little is known about their ecological role or their ability to confer tolerance. | |||
== | ==Responses to Drought Stress== | ||
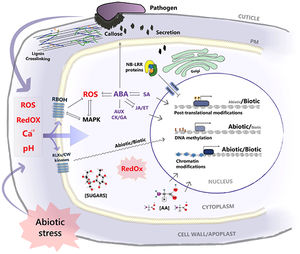
[[File:Kissoudis_et_al_(2014).jpg|thumb|300px|right| [http://www.frontiersin.org/files/Articles/90871/fpls-05-00207-r2/image_m/fpls-05-00207-g001.jpg <b> Figure 2. </b>] <b>Signaling pathways of abiotic and biotic stress at the cellular level.</b> Both types of stress factors affect the homeostasis of chemical signals such as Ca2+, ROS, and pH levels. Changes in these levels due to abiotic stress can affect the integrity of physical barriers against pathogens. Both abiotic and biotic stresses also share signaling nodes such as RBOHs (which produce ROS), [http://en.wikipedia.org/wiki/Mitogen-activated_protein_kinase MAPKs] (signal cascades that regulate stress and hormonal responses), receptor like kinases (RLKs) and other cell wall (CW) kinases which are used to regulate gene expression. The stress hormone [http://en.wikipedia.org/wiki/Abscisic_acid Abscisic acid] (ABA) is central to plants’ abiotic stress response. It inhibits hormones responsible for growth and development such as auxin (AUX), cytokinin (CK) and gibberellin (GA) and triggers increased generation of ROS as well as plant defense signaling ([http://en.wikipedia.org/wiki/Jasmonate JA]). Redox state and metabolite concentrations of sugars and amino acids (AA) regulate post-translational modifications. Changes in chromatin and DNA methylation status activate transcription factors to bind to stress responsive gene promoters to alter gene expression. [[#References |[13]]] ]] | |||
=== | ===Osmotic Adjustment=== | ||
Osmotic adjustment is a biochemical mechanism that helps plants tolerate drought and salt stress. [[#References |[15]]] It is an essential process that enables plants to maintain water uptake and metabolic processes.[[#References |[15]]] Increasing the levels of solutes such as water soluble sugars, prolin and other amino acids, and [http://en.wikipedia.org/wiki/Loline_alkaloid loline alkaloids] leads to a more negative [http://en.wikipedia.org/wiki/Osmotic_pressure osmotic potential].[[#References |[15]] This improves cell hydration so that the plant can maintain [http://en.wikipedia.org/wiki/Turgor_pressure turgor] in leaf tissue and in other metabolically active cells to stay alive. [[#References |[15]]]<br><br> | |||
The percentages of osmotically active carbohydrates such as glucose and fructose are known to vary inversely with soil moisture levels. [[#References |[8]]] [[#References |[16]]] Plants rely on carbohydrates during drought stress because their leaf area becomes insufficient to produce the energy the plant needs. [[#References |[16]]] Endophyte colonized and endophyte free plants would be expected to have similar levels of carbohydrates after the same drought stress, yet Arachelvaleta et al (1989) found that endophyte colonized plants recovered more quickly. [[#References |[16]]] They hypothesized that the high level of auxin production by fungal endophytes increased the efficiency of reserve carbohydrate mobilization and translocation and that this efficiency was more important than actual levels of available carbohydrates. [[#References |[16]]]<br><br> | |||
Additionally, Redman et al (2015) examined the osmotic concentrations in plants grown with and without heat-stress tolerant fungal endophytes. The differences in pattern between the two groups lead them to conclude that symbiotic plants do not rely solely on increasing [http://en.wikipedia.org/wiki/Osmolyte osmolyte] concentrations.[[#References |[6]]] <br> | |||
== | ===Reactive Oxygen Species=== | ||
[[ | [http://en.wikipedia.org/wiki/Abiotic_stress Abiotic stresses] such as drought result in the overproduction of [http://en.wikipedia.org/wiki/Reactive_oxygen_species reactive oxygen species] (ROS) [[#References |[10]]] These highly reactive metabolic products act as signaling molecules; however, when their levels are too high, they cause [http://en.wikipedia.org/wiki/Oxidative_stress oxidative stress] and damage proteins, lipids, and DNA. [[#References |[10]]] [[#References |[11]]] ROS control many plant processes such as growth, abiotic stress response, cell cycle, and programmed cell death because they influence the expression of genes. [[#References |[10]]]<br><br> | ||
When the stress hormone Abscisic acid (ABA) is highly induced by water stress, it triggers an increase in production of ROS. [[#References |[17]]] Plants have developed a complex antioxidant defense system to respond to increased ROS levels and employ scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), glutathione reductase (GR), and glutathione peroxidase (GPX) to adjust ROS homeostasis. [[#References |[17]]] [[#References |[18]]] Endophytes known to promote drought tolerance have also been found to have high levels of loline alkaloids. [[#References |[7]]] Future experiments could test if these are present in sufficient concentration to prevent the denaturation of macromolecules or reduce the number of reactive oxygen species. [[#References |[7]]] <br><br> | |||
Shi et al. (2012) compared natural variations in drought responses among three types of Bermudagrass. The variant Tifgreen performed best in the drought stress, showing the highest leaf water content and survival after 28 days of drought treatment. [[#References |[18]]] It had the highest levels of proline and sugar (osmolytes) and the lowest leaf electrolyte leakage, an indicator of cell membrane damage. [[#References |[18]]] It also had the highest antioxidant enzyme activity and the lowest levels of hydrogen peroxide (H2O2) and malondialdehyde (MDA), both indicators for ROS level and oxidative damage. [[#References |[18]]] <br> | |||
==Testing Endophyte Conferred Tolerances== | |||
[[File:tileshop.fcgi3.jpg|thumb|400px|right| [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3130040/figure/pone-0014823-g003/ <b> Figure 3. </b>] Effects of salt, cold and drought stress and the water usage in S and NS rice plants under laboratory conditions. Control plants (C) were grown in the absence of stress. <b> A. </b> SaltSym and NS plants were grown in 300 mM NaCl. <b> B. </b> TempSym1 and NS plants were grown at temperatures ranging from 5-20 degrees C. <b> C. </b> TempSym1 (TS1) and NS plants were not watered for the indicated number of days. <b>D.</b> Fluid usage of NS or S (SaltSym, TempSym1, and TempSym2) rice plants (N = 60) over a ten day period. All three symbionts used less fluid compared to NS plants (Duncan's multiple-range test SD≤7.51; P<0.05). [[#References |[6]]] ]] | |||
Redman et al (2015) isolated three Class 2 fungal endophytes from rice relatives to test how well they would confer abiotic stress tolerance to rice plants. <i>Fusarium culmorum</i> (SaltSym) was isolated from the coastal plant [http://en.wikipedia.org/wiki/Leymus_mollis <i>Leymus mollis</i>] which is exposed to high salt stress, while <i>Curvularia protuberant</i> (TempSym1 and 2) was isolated from [http://en.wikipedia.org/wiki/Dichanthelium_lanuginosum <i>Dichanthelium lanuginosum</i>] which grows in geothermal soils that can reach temperatures of up to 60 degrees Celcius.[[#References |[6]]] Rice plants with and without the fungal endophytes were grown in greenhouse conditions and subjected to treatments of salt, cold, and drought stress. | |||
<br>The study found that rice plants inoculated with the endophytes (S) showed no cost when grown in non-stressful conditions, but the percentage of colonized plants decreased from 100% to 65%.[[#References |[6]]] Plants grown under continual stress, however, maintained 100% colonization.[[#References |[6]]] All three endophytes treatments took 2-3 times longer to wilt than the non-colonized plants.[[#References |[6]]] While NS plants wilted after 3 days, SaltSym, TempSym1, and TempSym2 delayed wilt until after 9, 9, and 7 days respectively (Figure 3C).[[#References |[6]]] Over 90% of the NS plants had died after 5 days of drought stress, while 70% of S plants died after 12 days (Figure 3C).[[#References |[6]]] The water consumption of colonized plants grown in stressful environments decreased 20–30% while their growth rate, reproductive yield, and biomass increased (Figure 3D). [[#References |[6]]] Non-infected plants, on the other hand, lost shoot and root biomass when exposed to stress. [[#References |[6]]] ]] | |||
<br> | |||
All plant species used in this experiment were members of the family [http://en.wikipedia.org/wiki/Poaceae Poacea] but belonged to different subfamilies. [[#References |[6]]] While the mechanism remains unknown, the isolated fungal endophytes successfully conferred drought tolerance to the rice plants. This supports the idea that the necessary symbiotic communication between the fungi and the plant was conserved within the family. [[#References |[6]]] | |||
==Further Reading== | |||
Fungal endophytes have many potential applications in agriculture and conservation, yet there is still much that is not known about plant-fungi symbiosis and the mechanisms behind it. A more complete understanding of fungi’s role in plant community dynamics and ecosystem stability will inform conservation efforts around the world. While many fungal endophytes show habitat-adapted symbiosis, the fact that there is still lower biodiversity in high stress environments indicates that plants require more than just a fungal endophyte in order to be successful. [[#References |[6]]] Nevertheless, fungal endophytes’ ability to confer resistance to pathogens and deter insect herbivory gives them promise as alternative solutions to chemical pesticides and fungicides. Moreover, introducing fungal endophytes to crop may increase their drought tolerance by reducing their water consumption and increasing nutrient uptake. This may be used to increase crop yield in arid climates and mitigate the negative effects of climate change. | |||
[http://informahealthcare.com/doi/abs/10.3109/07388551.2013.800018 Khan, A. L., Hussain, J., Al-Harrasi, A., Al-Rawahi, A., & Lee, I. (2015). Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Critical Reviews in Biotechnology, 35(1), 62-74. doi:10.3109/07388551.2013.800018] | |||
This review examines how fungal endophytes re-program plant stress responses. As fungal endophytes are a "frequently overlooked component of crop | |||
ecology," the authors discuss mechanisms in plant-endophyte stress interactions, important endophyte-produced bioactive secondary metabolites such as the plant hormones Abscisic acid, Salicylic acid, Jasmonic acid, and [http://en.wikipedia.org/wiki/Gibberellin gibberellins] and suggest areas for future research in agricultural ecosystems. | |||
==References== | ==References== | ||
|[1] [https://books.google.com/books?hl=en&lr=&id=qLciOJaG0C4C&oi=fnd&pg=PP2&dq=Smith,+S.,+Read,+D.,+1997:+Mycorrhizal+symbiosis&ots=zptZgTSFpO&sig=svsWG48yCBBJHTr3-Gtx2mSdnfY#v=onepage&q=Smith%2C%20S.%2C%20Read%2C%20D.%2C%201997%3A%20Mycorrhizal%20symbiosis&f=false Smith, S., Read, D., 1997: Mycorrhizal symbiosis, 2nd edn., Academy Press, San Diego. ]<br> | |||
|[2] [http://link.springer.com/article/10.1007%2Fs00239-003-2564-9 Shu-Miaw C., Chien-Chang C., Hsin-Liang C., Wen-Hsiung L. (2004). Dating the Monocot–Dicot Divergence and the Origin of Core Eudicots Using Whole Chloroplast Genomes. Journal of Molecular Evolution. Volume 58, p. 424-441]<br> | |||
|[2] [http://link.springer.com/article/10.1007%2Fs00239-003-2564-9 Shu-Miaw C., Chien-Chang C., Hsin-Liang C., Wen-Hsiung L. | |[3] [http://www.sciencedirect.com/science/article/pii/S0169534797012305 Selosse, M., & Le Tacon, F. (1998). The land flora: A phototroph-fungus partnership? Trends in Ecology & Evolution, 13(1), 15-20. doi:http://dx.doi.org/10.1016/S0169-5347(97)01]<br> | ||
|[3] [http://www.sciencedirect.com/science/article/pii/S0169534797012305 Selosse, M | |[4] [http://ag.arizona.edu/mycoherb/arnoldlab/Rodriguez.pdf Rodriguez, R. J., White Jr, J. F., Arnold, A. E., & Redman, R. S. (2009). Fungal endophytes: Diversity and functional roles. New Phytologist, 182(2), 314-330. doi:10.1111/j.1469-8137.2009.02773.x]<br> | ||
|[4][http://ag.arizona.edu/mycoherb/arnoldlab/Rodriguez.pdf Rodriguez, R. J., | |[5] [http://www.sciencedirect.com/science/article/pii/S0031942206006522 Koulman, A., Lane, G. A., Christensen, M. J., Fraser, K., & Tapper, B. A. (2007). Peramine and other fungal alkaloids are exuded in the guttation fluid of endophyte-infected grasses. Phytochemistry, 68(3), 355-360. doi:http://dx.doi.org/10.1016/j.phytochem.2006.10.012] <br> | ||
|[5] [http://www.sciencedirect.com/science/article/pii/S0031942206006522 | |[6] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3130040/ Redman, R. S., Kim, Y. O., Woodward, C. J. D. A., Greer, C., Espino, L., Doty, S. L., & Rodriguez, R. J. (2011). Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. Plos One, 6(7), e14823. doi:10.1371/journal.pone.0014823]<br> | ||
Koulman, | |[7] [http://www.annualreviews.org/doi/full/10.1146/annurev.arplant.55.031903.141735?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed Schardl, C. L., Leuchtmann, A. & Spiering, M. J. (2004) Annu. Rev. Plant Biol. 55, 315-340. ]<br> | ||
|[6] [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3130040/ Redman, | |[8] [https://www.crops.org/publications/cs/abstracts/40/4/923 Malinowski DP, Belesky DP. (2000). Adaptations of endophtye-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science 40: 923–940.]<br> | ||
|[7] [http://www.annualreviews.org/doi/full/10.1146/annurev.arplant.55.031903.141735?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed Schardl, C. L., Leuchtmann, A. & Spiering, M. J. (2004) Annu. Rev. Plant Biol. 55, 315-340. ] | |[9] [http://www.sciencedirect.com/science/article/pii/S0098847206001237 Cheplick GP. (2006). Costs of fungal endophyte infection in Lolium perenne genotypes from eurasia and north africa under extreme resource limitation. Environmental and Experimental Botany 60: 202–210.]<br> | ||
|[8] [https://www.crops.org/publications/cs/abstracts/40/4/923 Malinowski DP, Belesky DP. 2000. Adaptations of endophtye-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science 40: 923–940.] | |[10] [http://www.sciencedirect.com/science/article/pii/S0981942810001798 Gill, S, Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909-930]<br> | ||
|[9] [http://www.sciencedirect.com/science/article/pii/S0098847206001237 Cheplick GP. 2006. Costs of fungal endophyte infection in Lolium perenne genotypes from eurasia and north africa under extreme resource limitation. Environmental and Experimental Botany 60: 202–210.] | |[11] [http://onlinelibrary.wiley.com/doi/10.1002/bies.20493/abstract Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I. and Laloi, C. (2006), Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays, 28: 1091–1101.]<br> | ||
|[12] [http://www.plantphysiol.org/content/156/2/726.full Jacobs, S., Zechmann, B., Molitor, A., Trujillo, M., Petutschnig, E., Likpa, V., Kogel, K-H., Schaefer, P. (2011). Broad-spectrum suppression of innate immunity is required for colonization of arabidopsis roots by the fungus piriformospora indica. Plant Physiology, 156(2), 726-740. doi:10.1104/pp.111.176446] <br> | |||
|[13] [http://journal.frontiersin.org/article/10.3389/fpls.2014.00207/full Kissoudis, C., van de Wiel, C., Visser, R. G. F., & van der Linden, G. (2014). Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Frontiers in Plant Science, 5, 207. doi:10.3389/fpls.2014.00207] <br> | |||
|[14] [http://informahealthcare.com/doi/abs/10.3109/07388551.2013.800018 Khan, A. L., Hussain, J., Al-Harrasi, A., Al-Rawahi, A., & Lee, I. (2015). Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Critical Reviews in Biotechnology, 35(1), 62-74. doi:10.3109/07388551.2013.800018] <br> | |||
|[15] [http://link.springer.com/chapter/10.1007%2F978-3-642-32653-0_8 Sanders, G., Arndt, S. Osm. 2012. Osmotic Adjustment Under Drought Conditions. Plant Responses to Drought Stress (199-299)] <br> | |||
|[16] [https://www.agronomy.org/publications/aj/abstracts/81/1/AJ0810010083?access=0&view=pdf ARACHEVALETA, M., BACON, C., HOVELAND, C., & RADCLIFFE, D. (1989). Effect of the tall fescue endophyte on plant-response to environmental-stress. Agronomy Journal, 81(1), 83-90.] <br> | |||
|[17] [http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0116646#pone.0116646.ref041 Cai, S., Jiang, G., Ye, N., Chu, Z., Xu, X., Zhang, J., & Zhu, G. (2015). A key ABA catabolic gene, OsABA8ox3, is involved in drought stress resistance in rice. Plos One, 10(2), e0116646. doi:10.1371/journal.pone.0116646]<br> | |||
|[18] [http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0053422 Shi, H., Wang, Y., Cheng, Z., Ye, T., & Chan, Z. (2012). Analysis of natural variation in bermudagrass (cynodon dactylon) reveals physiological responses underlying drought tolerance. Plos One, 7(12), e53422. doi:10.1371/journal.pone.0053422] <br> | |||
<!--Do not remove this line--> | <!--Do not remove this line--> | ||
Edited by | Edited by Sarah Barnes, a student of [http://www.jsd.claremont.edu/faculty/profile.asp?FacultyID=254 Nora Sullivan] in BIOL168L (Microbiology) in [http://www.jsd.claremont.edu/ The Keck Science Department of the Claremont Colleges] Spring 2014. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Nora Sullivan at the Claremont Colleges]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Nora Sullivan at the Claremont Colleges]] | ||
Latest revision as of 05:18, 15 April 2015
It has been estimated that over 80% of terrestrial plants form a symbiotic association with fungi.[1] Species of fungi that reside within living plant tissue without causing symptoms of disease in their host are known as fungal endophytes.[4] Fungal endophytes colonize a variety of both monocot and eudicot plants which suggests that this symbiosis predated the monocot-dicot split 140-150 million years ago.[2] It is also hypothesized that phototroph-fungi associations enabled plants to first colonize land.[3] This mutualistic association could have helped plants acclimate to new environmental stresses such as desiccation, increased exposure to solar radiation, and more extreme temperatures differences.[3]
Fungal endophytes remain an important component of today’s terrestrial ecosystems, and many enable their hosts to thrive in harsh environments. Studies show that fungal endophytes can enhance the drought, salt, and soil temperature tolerance of their host plant in addition to increasing resistance to parasitic fungi and herbivores.[4] With growing concerns about climate change and its effects on agriculture, learning about fungal endophyte conferred drought tolerance has become increasingly important. By influencing plant morphology, development, and physiological and biochemical responses to stress, fungal endophytes can induce mechanisms of drought avoidance, drought tolerance, and drought recovery in their hosts.[8]
Classes of Fungal Endophytes
Clavicipitaceous Endophytes
Class 1
Clavicipitaceous endophytes are associated with grasses.[4] They are typically found within the plant shoots and form systemic intercellular infections.[4] While they are often passed down in the seed through vertical transmission, they may also undergo horizontal transmission.[4] Many Class 1 endophytes produce alkaloids to protect their host plant from herbivory by insects and mammals, and studies have shown some Class 1 endophytes to confer drought and metal tolerance.[5][8] Neotyphodium coenophialum , for example, stimulates plants to develop more extensive root systems and longer and thinner root hairs which increases root surface area for improved water and mineral acquisition. [8]
Nonclavicipitaceous Endophytes
Nonclavicipitaceous endophytes are highly diverse and have been isolated from every major lineage of land plant, which includes nonvascular plants, ferns, conifers, and angiosperms.
Class 2
Class 2 endophytes are usually found in the roots, stem, or leaves of their hosts.[4] Like Class 1 endophytes, they can also be transmitted either vertically through the seed coat or horizontally. [4] They are unique in that they can confer habitat-specific stress tolerance to their hosts, and, as a result, they infect a higher percentage of plants in high-stress environments.[4] They often increase root and/or shoot biomass in their host.[4]
Class 3 and 4
Class 3 fungal endophytes colonize the shoot of plants while Class 4 endophytes colonize plant roots.[4] Both are horizontally transmitted.[4] Unlike the other classes, Class 3 endophyte colonizations are highly localized, and a diverse number of species can colonize an individual plant.[4] Few studies have been performed on Class 3 and 4 endophytes and little is known about their ecological role or their ability to confer tolerance.
Responses to Drought Stress
Osmotic Adjustment
Osmotic adjustment is a biochemical mechanism that helps plants tolerate drought and salt stress. [15] It is an essential process that enables plants to maintain water uptake and metabolic processes.[15] Increasing the levels of solutes such as water soluble sugars, prolin and other amino acids, and loline alkaloids leads to a more negative osmotic potential.[15 This improves cell hydration so that the plant can maintain turgor in leaf tissue and in other metabolically active cells to stay alive. [15]
The percentages of osmotically active carbohydrates such as glucose and fructose are known to vary inversely with soil moisture levels. [8] [16] Plants rely on carbohydrates during drought stress because their leaf area becomes insufficient to produce the energy the plant needs. [16] Endophyte colonized and endophyte free plants would be expected to have similar levels of carbohydrates after the same drought stress, yet Arachelvaleta et al (1989) found that endophyte colonized plants recovered more quickly. [16] They hypothesized that the high level of auxin production by fungal endophytes increased the efficiency of reserve carbohydrate mobilization and translocation and that this efficiency was more important than actual levels of available carbohydrates. [16]
Additionally, Redman et al (2015) examined the osmotic concentrations in plants grown with and without heat-stress tolerant fungal endophytes. The differences in pattern between the two groups lead them to conclude that symbiotic plants do not rely solely on increasing osmolyte concentrations.[6]
Reactive Oxygen Species
Abiotic stresses such as drought result in the overproduction of reactive oxygen species (ROS) [10] These highly reactive metabolic products act as signaling molecules; however, when their levels are too high, they cause oxidative stress and damage proteins, lipids, and DNA. [10] [11] ROS control many plant processes such as growth, abiotic stress response, cell cycle, and programmed cell death because they influence the expression of genes. [10]
When the stress hormone Abscisic acid (ABA) is highly induced by water stress, it triggers an increase in production of ROS. [17] Plants have developed a complex antioxidant defense system to respond to increased ROS levels and employ scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), glutathione reductase (GR), and glutathione peroxidase (GPX) to adjust ROS homeostasis. [17] [18] Endophytes known to promote drought tolerance have also been found to have high levels of loline alkaloids. [7] Future experiments could test if these are present in sufficient concentration to prevent the denaturation of macromolecules or reduce the number of reactive oxygen species. [7]
Shi et al. (2012) compared natural variations in drought responses among three types of Bermudagrass. The variant Tifgreen performed best in the drought stress, showing the highest leaf water content and survival after 28 days of drought treatment. [18] It had the highest levels of proline and sugar (osmolytes) and the lowest leaf electrolyte leakage, an indicator of cell membrane damage. [18] It also had the highest antioxidant enzyme activity and the lowest levels of hydrogen peroxide (H2O2) and malondialdehyde (MDA), both indicators for ROS level and oxidative damage. [18]
Testing Endophyte Conferred Tolerances
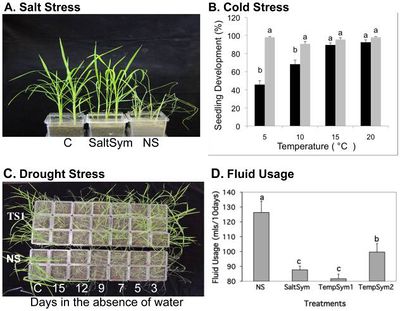
Redman et al (2015) isolated three Class 2 fungal endophytes from rice relatives to test how well they would confer abiotic stress tolerance to rice plants. Fusarium culmorum (SaltSym) was isolated from the coastal plant Leymus mollis which is exposed to high salt stress, while Curvularia protuberant (TempSym1 and 2) was isolated from Dichanthelium lanuginosum which grows in geothermal soils that can reach temperatures of up to 60 degrees Celcius.[6] Rice plants with and without the fungal endophytes were grown in greenhouse conditions and subjected to treatments of salt, cold, and drought stress.
The study found that rice plants inoculated with the endophytes (S) showed no cost when grown in non-stressful conditions, but the percentage of colonized plants decreased from 100% to 65%.[6] Plants grown under continual stress, however, maintained 100% colonization.[6] All three endophytes treatments took 2-3 times longer to wilt than the non-colonized plants.[6] While NS plants wilted after 3 days, SaltSym, TempSym1, and TempSym2 delayed wilt until after 9, 9, and 7 days respectively (Figure 3C).[6] Over 90% of the NS plants had died after 5 days of drought stress, while 70% of S plants died after 12 days (Figure 3C).[6] The water consumption of colonized plants grown in stressful environments decreased 20–30% while their growth rate, reproductive yield, and biomass increased (Figure 3D). [6] Non-infected plants, on the other hand, lost shoot and root biomass when exposed to stress. [6] ]]
All plant species used in this experiment were members of the family Poacea but belonged to different subfamilies. [6] While the mechanism remains unknown, the isolated fungal endophytes successfully conferred drought tolerance to the rice plants. This supports the idea that the necessary symbiotic communication between the fungi and the plant was conserved within the family. [6]
Further Reading
Fungal endophytes have many potential applications in agriculture and conservation, yet there is still much that is not known about plant-fungi symbiosis and the mechanisms behind it. A more complete understanding of fungi’s role in plant community dynamics and ecosystem stability will inform conservation efforts around the world. While many fungal endophytes show habitat-adapted symbiosis, the fact that there is still lower biodiversity in high stress environments indicates that plants require more than just a fungal endophyte in order to be successful. [6] Nevertheless, fungal endophytes’ ability to confer resistance to pathogens and deter insect herbivory gives them promise as alternative solutions to chemical pesticides and fungicides. Moreover, introducing fungal endophytes to crop may increase their drought tolerance by reducing their water consumption and increasing nutrient uptake. This may be used to increase crop yield in arid climates and mitigate the negative effects of climate change.
This review examines how fungal endophytes re-program plant stress responses. As fungal endophytes are a "frequently overlooked component of crop ecology," the authors discuss mechanisms in plant-endophyte stress interactions, important endophyte-produced bioactive secondary metabolites such as the plant hormones Abscisic acid, Salicylic acid, Jasmonic acid, and gibberellins and suggest areas for future research in agricultural ecosystems.
References
|[1] Smith, S., Read, D., 1997: Mycorrhizal symbiosis, 2nd edn., Academy Press, San Diego.
|[2] Shu-Miaw C., Chien-Chang C., Hsin-Liang C., Wen-Hsiung L. (2004). Dating the Monocot–Dicot Divergence and the Origin of Core Eudicots Using Whole Chloroplast Genomes. Journal of Molecular Evolution. Volume 58, p. 424-441
|[3] Selosse, M., & Le Tacon, F. (1998). The land flora: A phototroph-fungus partnership? Trends in Ecology & Evolution, 13(1), 15-20. doi:http://dx.doi.org/10.1016/S0169-5347(97)01
|[4] Rodriguez, R. J., White Jr, J. F., Arnold, A. E., & Redman, R. S. (2009). Fungal endophytes: Diversity and functional roles. New Phytologist, 182(2), 314-330. doi:10.1111/j.1469-8137.2009.02773.x
|[5] Koulman, A., Lane, G. A., Christensen, M. J., Fraser, K., & Tapper, B. A. (2007). Peramine and other fungal alkaloids are exuded in the guttation fluid of endophyte-infected grasses. Phytochemistry, 68(3), 355-360. doi:http://dx.doi.org/10.1016/j.phytochem.2006.10.012
|[6] Redman, R. S., Kim, Y. O., Woodward, C. J. D. A., Greer, C., Espino, L., Doty, S. L., & Rodriguez, R. J. (2011). Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. Plos One, 6(7), e14823. doi:10.1371/journal.pone.0014823
|[7] Schardl, C. L., Leuchtmann, A. & Spiering, M. J. (2004) Annu. Rev. Plant Biol. 55, 315-340.
|[8] Malinowski DP, Belesky DP. (2000). Adaptations of endophtye-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Science 40: 923–940.
|[9] Cheplick GP. (2006). Costs of fungal endophyte infection in Lolium perenne genotypes from eurasia and north africa under extreme resource limitation. Environmental and Experimental Botany 60: 202–210.
|[10] Gill, S, Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiology and Biochemistry 48: 909-930
|[11] Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I. and Laloi, C. (2006), Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays, 28: 1091–1101.
|[12] Jacobs, S., Zechmann, B., Molitor, A., Trujillo, M., Petutschnig, E., Likpa, V., Kogel, K-H., Schaefer, P. (2011). Broad-spectrum suppression of innate immunity is required for colonization of arabidopsis roots by the fungus piriformospora indica. Plant Physiology, 156(2), 726-740. doi:10.1104/pp.111.176446
|[13] Kissoudis, C., van de Wiel, C., Visser, R. G. F., & van der Linden, G. (2014). Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Frontiers in Plant Science, 5, 207. doi:10.3389/fpls.2014.00207
|[14] Khan, A. L., Hussain, J., Al-Harrasi, A., Al-Rawahi, A., & Lee, I. (2015). Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Critical Reviews in Biotechnology, 35(1), 62-74. doi:10.3109/07388551.2013.800018
|[15] Sanders, G., Arndt, S. Osm. 2012. Osmotic Adjustment Under Drought Conditions. Plant Responses to Drought Stress (199-299)
|[16] ARACHEVALETA, M., BACON, C., HOVELAND, C., & RADCLIFFE, D. (1989). Effect of the tall fescue endophyte on plant-response to environmental-stress. Agronomy Journal, 81(1), 83-90.
|[17] Cai, S., Jiang, G., Ye, N., Chu, Z., Xu, X., Zhang, J., & Zhu, G. (2015). A key ABA catabolic gene, OsABA8ox3, is involved in drought stress resistance in rice. Plos One, 10(2), e0116646. doi:10.1371/journal.pone.0116646
|[18] Shi, H., Wang, Y., Cheng, Z., Ye, T., & Chan, Z. (2012). Analysis of natural variation in bermudagrass (cynodon dactylon) reveals physiological responses underlying drought tolerance. Plos One, 7(12), e53422. doi:10.1371/journal.pone.0053422
Edited by Sarah Barnes, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.
