Gram staining: Difference between revisions
From MicrobeWiki, the student-edited microbiology resource
mNo edit summary |
No edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Short pages]] | |||
{{Uncurated}} | {{Uncurated}} | ||
[[Image:20100926_180209_DentalPlaque.jpg|thumb|right|500px|A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. The larger red cells are something else, not bacteria.<br/>Numbered ticks are 11 µM apart.<br/>Photograph by [[User:Blaylock|Bob Blaylock]]]] | [[Image:20100926_180209_DentalPlaque.jpg|thumb|right|500px|A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. The larger red cells are something else, not bacteria.<br/>Numbered ticks are 11 µM apart.<br/>Photograph by [[User:Blaylock|Bob Blaylock]]]] | ||
[[Image:20100829_233446_Bacteria.jpg|thumb|right|500px|A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. This image shows predominantly Gram-negative cocci, with a few particularly long Gram-negative bacilli.<br/>Numbered ticks are 11 µM apart.<br/>Photograph by [[User:Blaylock|Bob Blaylock]]]] | [[Image:20100829_233446_Bacteria.jpg|thumb|right|500px|A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. This image shows predominantly Gram-negative cocci, with a few particularly long Gram-negative bacilli.<br/>Numbered ticks are 11 µM apart.<br/>Photograph by [[User:Blaylock|Bob Blaylock]]]] | ||
[[Image:20100926_175021_DentalPlaque.jpg|thumb|right|500px|A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. A more even mix than the previous image, but the colors aren't quite as vivid as I'd like.<br/>Numbered ticks are 11 µM apart.<br/>Photograph by [[User:Blaylock|Bob Blaylock]]]] | [[Image:20100926_175021_DentalPlaque.jpg|thumb|right|500px|A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. A more even mix than the previous image, but the colors aren't quite as vivid as I'd like.<br/>Numbered ticks are 11 µM apart.<br/>Photograph by [[User:Blaylock|Bob Blaylock]]]] | ||
Gram staining is a process for staining bacteria, and differentiating them into two major groups: Gram-positive (which stain purple | Gram staining is a process for staining bacteria, and differentiating them into two major groups: Gram-positive (which stain purple or violet under this procedure) and Gram-negative (which stain pink). | ||
The word “Gram” is always to be capitalized, as it is the name of Hans Christian Gram, who invented this method, and published it in 1884. | The word “Gram” is always to be capitalized, as it is the name of Hans Christian Gram, who invented this method, and published it in 1884. | ||
Latest revision as of 14:37, 28 September 2015
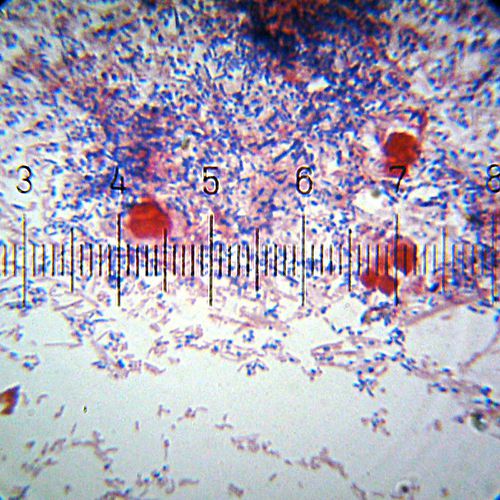
A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. The larger red cells are something else, not bacteria.
Numbered ticks are 11 µM apart.
Photograph by Bob Blaylock
Numbered ticks are 11 µM apart.
Photograph by Bob Blaylock
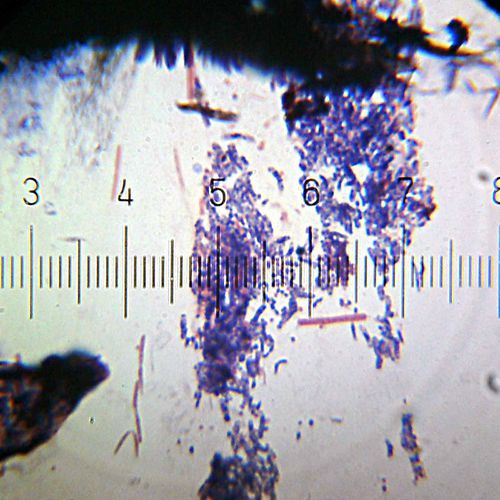
A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. This image shows predominantly Gram-negative cocci, with a few particularly long Gram-negative bacilli.
Numbered ticks are 11 µM apart.
Photograph by Bob Blaylock
Numbered ticks are 11 µM apart.
Photograph by Bob Blaylock
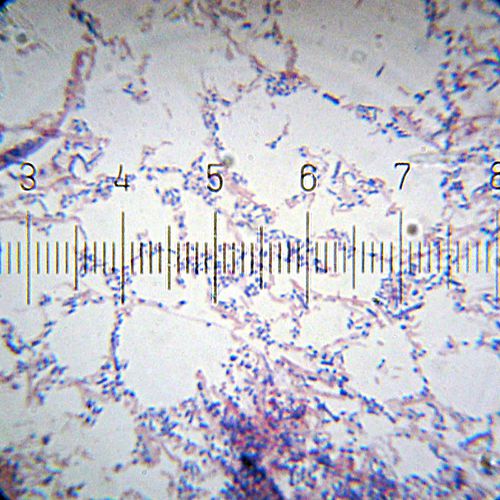
A Gram-stained sample showing both Gram-positive (purple) and Gram-negative (pink) bacteria. A more even mix than the previous image, but the colors aren't quite as vivid as I'd like.
Numbered ticks are 11 µM apart.
Photograph by Bob Blaylock
Numbered ticks are 11 µM apart.
Photograph by Bob Blaylock
Gram staining is a process for staining bacteria, and differentiating them into two major groups: Gram-positive (which stain purple or violet under this procedure) and Gram-negative (which stain pink).
The word “Gram” is always to be capitalized, as it is the name of Hans Christian Gram, who invented this method, and published it in 1884.
Procedure
- Spread a thin film of the sample on a slide. If necessary, allow it to air-dry.
- Heat fix the sample by holding the slide over a flame, moving it around, until the slide is noticeably quite warm, but not until it is too hot to hold in your fingers.
- Flood the sample with crystal violet solution. Let this sit for approximately a minute, then rinse under running water.
- Flood the sample with Gram's iodine solution. Again, allow to sit for about a minute, then rinse under running water.
- The decolorizing step is the most critical, and most subject, it seems, to individual variation. Too aggressive, and you'll cause Gram-positive bacteria to falsely stain as Gram-negative. Not aggressive enough, and you'll fail to remove the crystal violet from the Gram-negative bacteria, causing them to appear as Gram-positive. What follows is what I have found, from trial and error, seems to work best for me.
- Flood the sample with a roughly half-and-half mixture of acetone and denatured ethanol, allow to sit for just a few seconds, then rinse it off with running water.
- Hold the slide vertically, and run some pure denatured ethanol down the slide, across the sample. Do this until you no longer see any violet running from the sample. Rinse with running water.
- Flood the sample with safranin O. Allow to sit for about a minute, then rinse with running water.
- Allow to air-dry. I usually use some “canned air” to blow it dry faster.
