Cryptococcus neoformans: Difference between revisions
No edit summary |
|||
| (32 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Conway}} | |||
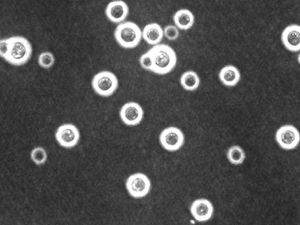
[[Image:cneoindiaink.jpg|thumb|300px|right|India ink stain of Cryptococcus neosporans showing the presence of a capsule. From: http://sequence-www.stanford.edu/group/C.neoformans/]] | [[Image:cneoindiaink.jpg|thumb|300px|right|India ink stain of Cryptococcus neosporans showing the presence of a capsule. From: http://sequence-www.stanford.edu/group/C.neoformans/]] | ||
| Line 16: | Line 16: | ||
===Description=== | ===Description=== | ||
''Cryptococcus neoformans'' is a spherical, encapsulated, non-myceliated, non-fermenting fungal cell [[#References|[ | ''Cryptococcus neoformans'' is a spherical, encapsulated, non-myceliated, non-fermenting fungal cell [[#References|[1]]]. Among pathogenic fungi, ''C. neoformans'' is unique in that it possesses a mucinous capsule. Small-sized basidiospores (1.8 to 3.0 μm) can turn into yeast cells, the form preferred at 37°C, or can form dikaryotic hyphae which are favoured at 24°C [[#References|[2]]]. ''C. neoformans'' causes fatal meningitis primarily in immunosuppressed humans [[#References|[3]]]. It is usually found in tissues in the yeast form. Besides a prevalent asexual life cycle, ''C. neoformans'' also presents a bipolar mating cycle with two mating types, MATa and MATα, with the latter being the most prevalently isolated from hosts and the environment [[#References|[3]]]. ''Filobasidiella neoformans'' is the teleomorph (sexual state). During sexual recombination, either filaments with clamp connections or basidiospores are produced. Recombinant basidiospores are produced via same-sex mating and are thought to be the propagules responsible for infection. | ||
==Genome structure== | ==Genome structure== | ||
| Line 24: | Line 24: | ||
===Transmission=== | ===Transmission=== | ||
An environmental survey found that ''Cryptococcus neoformans'' was isolated from pigeon and other bird excreta and less frequently in other environmental and veterinary (cat, dog, horse, sheep, cow) sources [[#Reference|[ | An environmental survey found that ''Cryptococcus neoformans'' was isolated from pigeon and other bird excreta and less frequently in other environmental and veterinary (cat, dog, horse, sheep, cow) sources [[#Reference|[3]]]. The organism exists as both free-living and in association with a variety of hosts [[#Reference|[4]]]. The primary mode of transmission is inhalation of spores from the environment, affecting both humans and other domestic and wild animals. Transmission from humans and other animals has not been documented. | ||
===Infection and incubation=== | ===Infection and incubation=== | ||
Once ''C. neoformans'' spores have been inhaled, infection begins. One key requirement for its persistence is the ability to adapt to the host's physiological conditions. In strains where physiological conditions do not inhibit growth, pathogenicity is determined by the ''CNA1'' gene encoding calcineurin A. Calcineurin A is a Ca2+-calmodulin-regulated protein phosphatase activated by a stress response. Calcineurin A dephosphorylates a group of proteins, allowing for growth at 37°C. Deletion of ''CNA1'' also demonstrates a decreased sensitivity to elevated CO2 and alkaline pH characteristic of host tissues. This indicates that the calcineurin pathway regulates several crucial survival responses. | Once ''C. neoformans'' spores have been inhaled, infection begins. One key requirement for its persistence is the ability to adapt to the host's physiological conditions. In strains where physiological conditions do not inhibit growth, pathogenicity is determined by the ''CNA1'' gene encoding calcineurin A [[#References|[4]]]. Calcineurin A is a Ca2+-calmodulin-regulated protein phosphatase activated by a stress response. Calcineurin A dephosphorylates a group of proteins, allowing for growth at 37°C. Deletion of ''CNA1'' also demonstrates a decreased sensitivity to elevated CO2 and alkaline pH characteristic of host tissues [[#References|[4]]]. This indicates that the calcineurin pathway regulates several crucial survival responses. | ||
===Cerebral infection and survival=== | ===Cerebral infection and survival=== | ||
Different routes of infection have been demonstrated in different models. Paracellular and transcellular mechanisms of crossing the endothelial microcapillary wall, as well as in a phagocytosed form using monocytes as "Trojan horses" have been demonstrated as a means of invasion of the central nervous system. Presently it is not clear which mode of infection is preferred, though the microbe's ability to utilize different routes likely contributes to its high propensity to affect the CNS. In the brain, adaptations associated with persistence in the brain include a shift from high (lungs) to low (brain) oxygen and opposition to local host defenses. | Different routes of infection have been demonstrated in different models. Paracellular and transcellular mechanisms of crossing the endothelial microcapillary wall, as well as in a phagocytosed form using monocytes as "Trojan horses" have been demonstrated as a means of invasion of the central nervous system [[#References|[4]]]. Presently it is not clear which mode of infection is preferred, though the microbe's ability to utilize different routes likely contributes to its high propensity to affect the CNS. In the brain, adaptations associated with persistence in the brain include a shift from high (lungs) to low (brain) oxygen and opposition to local host defenses. | ||
====Paracellular route==== | ====Paracellular route==== | ||
Following sequestration in cerebral capillaries, damage to endothelium is caused by changes in cryptococcal capsule structure and cell size. ''C. neoformans'' can also utilize the host plasminogen system to enhance degradation and invasion of tissues and penetrate the blood-brain barrier. Studies have linked the importance of cryptococcal urease to CNS invasion. | |||
====Transcellular route==== | ====Transcellular route==== | ||
| Line 53: | Line 53: | ||
====App1==== | ====App1==== | ||
Secreted cryptococcal protein App1 inhibits phagocytosis through a complement-mediated mechanism, and deletion of App1 improves phagocytosis by alveolar macrophages. App1 blocks complement 3 and complement 2 receptors (CR3 and CR2), interfering with phagocytosis [[#Reference|[ | Secreted cryptococcal protein App1 inhibits phagocytosis through a complement-mediated mechanism, and deletion of App1 improves phagocytosis by alveolar macrophages. App1 blocks complement 3 and complement 2 receptors (CR3 and CR2), interfering with phagocytosis [[#Reference|[4]]] | ||
====Modulation of the adaptive immune response==== | ====Modulation of the adaptive immune response==== | ||
| Line 59: | Line 59: | ||
==Epidemiology== | ==Epidemiology== | ||
A close correlation between the occurrence of ''C. neoformans'' and pigeon droppings has been shown [[#References|[ | [[Image:hivcryptoneo.jpg|thumb|300px|right|Global burden of HIV-related cryptococcal meningitis. From: http://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/statistics.html]] | ||
A close correlation between the occurrence of ''C. neoformans'' and pigeon droppings has been shown [[#References|[5]]]. ''C. neoformans var. grubii'' has a worldwide distribution with the most cases being reported in the United States and Australia, while ''C. neoformans var. neoformans'' is is mostly restricted to France, Italy, and Denmark [[#References|[6]]]. Among immmunocompetent individuals, groups at risk are the elderly and those using corticosteroids [[#References|[5]]]. In the United States, only 25% of infections are reported as symptomatic among immunocompetent individuals [[#References|[4]]]. Among HIV/AIDS patients, it is estimated that approximately 957,900 cases occur each year, resulting in nearly 625,000 deaths [[#References|[3]]]. Worldwide, 7-10% of patients with AIDS are affected [[#References|[3]]]. AIDS-associated cryptococcosis accounts for 50% of cryptococcal infections reported worldwide, usually occurring in HIV patients when their CD4 lymphocyte count falls below 200/mm3. In sub-Saharan Africa, the mortality rate is estimated to be between 50-70% . In developed countries such as the United States where therapy is available, the mortality rate is as low as 12% [[#References|[3]]]. | |||
==Clinical features== | ==Clinical features== | ||
''Cryptococcus neoformans'' most often infects the lungs or the central nervous system, but it can also affect other parts of the body as well [[#References|[7]]]. Symptoms are dependent on which area of the body is infected. In immunocompetent individuals, infections are usually asymptomatic and do not spread past the lungs. | |||
===Lungs=== | |||
''C. neoformans'' infection in the lung causes typical pneumonia-like symptoms, including shortness of breath, coughing, and fever. | |||
===Central nervous system=== | |||
Cryptococcal meningitis is the result of an infection spreading past the blood-brain barrier, allowing access to the central nervous system. Cryptococcal meningitis is a serious illness, commonly associated with swelling and irritation of the brain and spinal cord. Neurological symptoms do not typically occur until days or weeks after infection and include fever, hallucinations, headache, confusion, nausea and vomiting, light sensitivity, stiff neck, and blurred vision [[#References|[8]]]. | |||
==Diagnosis== | ==Diagnosis== | ||
Diagnostic features are difficult to distinguish, as visible findings such as skin, pulmonary, or bone lesions seen on X-ray are also characteristic of histoplasmosis, toxoplasmosis, and tuberculosis. A CT scan or MRI of the brain may show areas of possible infection, although many other diseases present similar findings as well. Serological testing of spinal fluid may be used if the patient is suspected to have an infection. Definitive diagnosis depends on isolation of the fungus from the patient's tissue or bodily fluids or identification from tissue biopsy samples. Further analysis, such as PCR, may distinguish whether the infection is caused by ''C. neoformans'' or ''C. gattii'' [[#Reference|[ | Diagnostic features are difficult to distinguish, as visible findings such as skin, pulmonary, or bone lesions seen on X-ray are also characteristic of histoplasmosis, toxoplasmosis, and tuberculosis. A CT scan or MRI of the brain may show areas of possible infection, although many other diseases present similar findings as well. Serological testing of spinal fluid may be used if the patient is suspected to have an infection. Definitive diagnosis depends on isolation of the fungus from the patient's tissue or bodily fluids or identification from tissue biopsy samples. Further analysis, such as PCR, may distinguish whether the infection is caused by ''C. neoformans'' or ''C. gattii'' [[#Reference|[9]]]. | ||
If the infection progresses to meningitis, more diagnostic features are present. These include faster heart rate, fever, mental state changes, and stiff neck. If meningitis is suspected, a lumbar puncture is an important test for removal of a sample of cerebrospinal fluid for further inspection. | |||
==Treatment== | ==Treatment== | ||
In healthy individuals, treatment is usually not necessary. Still, it is recommended to receive check-ups for up to monitor any spreading of the infection. If there are lung lesions or the disease spreads, antifungals such as fluconazole, itraconazole, and voriconazole may be prescribed to clear the infection. Patients with severe lung infections or infections of the central nervous system are recommended to take amphotericin B in combination with flucytosine. In some cases, surgery may be necessary to remove fungal growths (cryptococcomonas) [[#Reference|[ | In healthy individuals, treatment is usually not necessary. Still, it is recommended to receive check-ups for up to monitor any spreading of the infection. If there are lung lesions or the disease spreads, antifungals such as fluconazole, itraconazole, and voriconazole may be prescribed to clear the infection. Patients with severe lung infections or infections of the central nervous system are recommended to take amphotericin B in combination with flucytosine. In some cases, surgery may be necessary to remove fungal growths (cryptococcomonas) [[#Reference|[7]]]. | ||
==Prevention== | ==Prevention== | ||
As ''Cryptococcus neosporans'' is spread throughout the environment via bird droppings, it is difficult to establish recommendations for prevention. Immunocompromised individuals should avoid areas known to be contaminated with bird droppings and should avoid contact with birds. | |||
==Host immune response== | ==Host immune response== | ||
Despite interaction with contaminated areas, most immunocompetent individuals are able to contain the fungus or maintain the yeast in latent state. Besides barriers such as skin and nasal mucosa, a variety of innate factors are able to interfere with the establishment of infection. Human serum and saliva has been shown to exhibit anticryptococcal activity [[#References|[10]]]. However, the complement system and phagocytes are the main responses to infection. | |||
===Complement=== | |||
The complement system leads to inflammation and recruitment of phagocytic effector cells as well as the formation of the membrane attack complex. Animal model systems and human patients have demonstrated the importance of the complement system [[#References|[10]]]. Guinea pigs treated with cobra venom to deplete complement components had shorter survival time, and mice deficient in C5 are more susceptible to infection and succumb three times quicker than C5 positive mice [[#References|[11]]]. The survival time of C4-deficient guinea pigs is similar to that of normal guinea pigs, suggesting that the C4-independent alternative pathway is a viable response to infection. The lectin pathway of the complement system is dependent on capsulation of ''Cryptococcus neoformans'' as the capsule inhibits the binding of mannan-binding lectin. | |||
===Phagocytic effector cells=== | |||
As the first line of defense against infection in the bloodstream, the complement system achieves certain preparations for further response through opsonization and phagocytic effector cell recruitment. In humans, neutrophils and macrophages are recruited for phagocytosis. Phagocytosis is triggered through direct contact with conserved pathogenic structures such as components of the cryptococcal capsule or through indirect recognition of antibody-opsonized cells. Dendritic cells function as major antigen-presenting cells and also initiate cell-mediated immunity. T-cell response by dendritic cells is much more efficient than by alveolar or peritoneal macrophages. At the site of infection, the number of neutrophil granulocytes increases as well. Oxidative bursts by neutrophils also act to remove the infection, although these bursts also damage surrounding host cells. Considering the still low number of neutrophils present at the site of infection, it is possible that neutrophils provide more of a regulatory rather than antimicrobial role. Recent studies, however, have suggested that macrophages may actually be harmful in infections as ''C. neoformans'' has developed several exploitative mechanisms to utilize macrophages [[#References|[12]]]. | |||
===Cell-mediated immunity=== | |||
Individuals with HIV, leukemia, lymphoma, or on extensive corticosteroid treatment all belong to the group at highest risk for symptomatic cryptococcal infections [[#References|[3]]]. The common feature of each of these groups is a defect in cell-mediated immunity, demonstrating its significance in combating the infection. Cell-mediated immunity refers to the killing of pathogens through direct killing via cytotoxic effects or indirect killing via NK cells and T lymphocytes. NK, CD4+, and CD8+ cells all act against the infection, and the secretion of granulysin and perforin are effective in cryptococcal permeabilization and lysis. In HIV patients, the low CD4+ count prevents secretion of these molecules. | |||
==References== | ==References== | ||
1. '''Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, et al.''' 2010. ''Cryptococcal Cell Morphology Affects Host Cell Interactions and Pathogenicity.'' PLoS Pathog | |||
2. '''Karkowska-Kuleta, J, Rapala-Kozik, M, Kozik, A.''' 2009. ''Fungi pathogenic to humans: Molecular bases of virulence of ''Candida albicans'', ''Cryptococcus neoformans'' and ''Aspergillus fumigatus''. Acta Biochimica Polonica'' | |||
3. '''Cogliati M.''' 2013. ''Global Molecular Epidemiology of ''Cryptococcus neoformans'' and ''Cryptococcus gattii'': An Atlas of the Molecular Types.'' Scientifica | |||
4. '''Olszewski MA, Zhang Y, Huffnagle GB.''' 2010. ''Mechanisms of Cryptococcal Virulence and Persistence.'' Future Microbiology | |||
5. '''Lonian RD.''' 1965. ''The Effects of Steroids on the Virulence of ''Cryptococcus neoformans''.''Oklahoma State University. | |||
6. '''The University of Adelaide.''' ''''Cryptococcus neoformans.'''' Mycology Online. | |||
7. http://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/ | |||
8. http://www.nytimes.com/health/guides/disease/cryptococcosis/overview.html | |||
9. http://www.emedicinehealth.com/cryptococcosis/article_em.htm | |||
10. http://ec.asm.org/content/9/6/835.full | |||
11. '''Rhodes JC, Wicker LS, Urba WJ.''' 1980. ''Genetic control of susceptibility to ''Cryptococcus neoformans'' in mice.'' Infect. Immun. | |||
12. '''Kechichian TB, Shea J, Del Poeta M.''' 2007. ''Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of ''Cryptococcus neoformans'' in immunodeficient mice.'' Infect. Immun. | |||
Created by Chris Vu, student of Tyrell Conway at the University of Oklahoma. | Created by Chris Vu, student of Tyrell Conway at the University of Oklahoma. | ||
Latest revision as of 14:33, 11 February 2016

Etiology/Bacteriology
Higher order taxa
Eukaryota (Kingdom); Fungi (Domain); Basidiomycota (Phylum); Tremellomycetes (Class); Tremellales (Order); Tremellaceae (Family); Cryptococcus (Genus)
Species
C. neoformans v. grubii (serotype A), C. neoformans v. neoformans (serotype D). Its teleomorph is Filobasidiella neoformans. A third variety, once known as C. neoformans v. gattii, is now considered a distinct species, Cryptococcus gattii.
|
NCBI: Taxonomy Genome: Cryptotoccus neoformans |
Description
Cryptococcus neoformans is a spherical, encapsulated, non-myceliated, non-fermenting fungal cell [1]. Among pathogenic fungi, C. neoformans is unique in that it possesses a mucinous capsule. Small-sized basidiospores (1.8 to 3.0 μm) can turn into yeast cells, the form preferred at 37°C, or can form dikaryotic hyphae which are favoured at 24°C [2]. C. neoformans causes fatal meningitis primarily in immunosuppressed humans [3]. It is usually found in tissues in the yeast form. Besides a prevalent asexual life cycle, C. neoformans also presents a bipolar mating cycle with two mating types, MATa and MATα, with the latter being the most prevalently isolated from hosts and the environment [3]. Filobasidiella neoformans is the teleomorph (sexual state). During sexual recombination, either filaments with clamp connections or basidiospores are produced. Recombinant basidiospores are produced via same-sex mating and are thought to be the propagules responsible for infection.
Genome structure
Most isolates of Cryptococcus neoformans are haploid. The size of its genome is approximately 19 Mb with 14 chromosomes. C. neoformans has a defined sexual cycle involving mating between cells of the MATa and MATα types. Thus, classical genetic approaches can be applied to study this organism.
Pathogenesis
Transmission
An environmental survey found that Cryptococcus neoformans was isolated from pigeon and other bird excreta and less frequently in other environmental and veterinary (cat, dog, horse, sheep, cow) sources [3]. The organism exists as both free-living and in association with a variety of hosts [4]. The primary mode of transmission is inhalation of spores from the environment, affecting both humans and other domestic and wild animals. Transmission from humans and other animals has not been documented.
Infection and incubation
Once C. neoformans spores have been inhaled, infection begins. One key requirement for its persistence is the ability to adapt to the host's physiological conditions. In strains where physiological conditions do not inhibit growth, pathogenicity is determined by the CNA1 gene encoding calcineurin A [4]. Calcineurin A is a Ca2+-calmodulin-regulated protein phosphatase activated by a stress response. Calcineurin A dephosphorylates a group of proteins, allowing for growth at 37°C. Deletion of CNA1 also demonstrates a decreased sensitivity to elevated CO2 and alkaline pH characteristic of host tissues [4]. This indicates that the calcineurin pathway regulates several crucial survival responses.
Cerebral infection and survival
Different routes of infection have been demonstrated in different models. Paracellular and transcellular mechanisms of crossing the endothelial microcapillary wall, as well as in a phagocytosed form using monocytes as "Trojan horses" have been demonstrated as a means of invasion of the central nervous system [4]. Presently it is not clear which mode of infection is preferred, though the microbe's ability to utilize different routes likely contributes to its high propensity to affect the CNS. In the brain, adaptations associated with persistence in the brain include a shift from high (lungs) to low (brain) oxygen and opposition to local host defenses.
Paracellular route
Following sequestration in cerebral capillaries, damage to endothelium is caused by changes in cryptococcal capsule structure and cell size. C. neoformans can also utilize the host plasminogen system to enhance degradation and invasion of tissues and penetrate the blood-brain barrier. Studies have linked the importance of cryptococcal urease to CNS invasion.
Transcellular route
Studies indicate that C. neoformans is capable of migrating through the endothelium monolayer through transcytosis. The interaction between hyaluronic acid produced by C. neoformans and endothelium CD44 is required for optimal contact to induce transcytosis. Transcytosis is accompanied by activation of protein kinase C-a and is co-localization with actin filaments. This is followed by cytoskeletal arrangements which contribute to the migration of C. neoformans.
"Trojan horse" route
The ability for C. neoformans to utilize monocytes as a passenger allows it to migrate to the brain while also depleting the host of its blood phagocytes.
Virulence factors
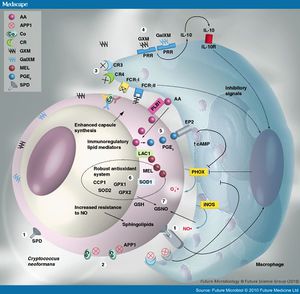
Cell enlargement
The size of cells are typically 5-10µm in diameter. However, cell enlargement has been observed in vivo, producing cells up to 100µm in diameter, making it rare for phagocytosis to occur. It is thought that these morphological changes affect pathogenicity via reducing phagocytosis, increasing resistance to oxidative and nitrosative stress, correlating with reduced penetration of the central nervous system. Cell enlargement is stimulated by coinfection with strains of the opposite mating type, and ste3aΔ pheromone receptor mutant strains had reduced cell enlargement. DNA analysis shows that these enlarged cells are polyploid, uninucleate, and capable of producing daughter cells in vivo.
Capsule
The capsule itself can mediate immune evasion through several different mechanisms. The capsule, made up of polysaccharides rich in mannan residues, is strongly hydrophobic in nature. This helps prevent contact with exogenous factors and prevents recognition by some cells of the immune response. It also prevents efficient antibody binding and the resultant activation of the complement system via classical pathway. The capsule is also capable of interacting directly with components of the immune system, namely glucuronoxylomannan (GXM) and galactoxylomannan, both of which are capable of inducing leukocyte activation and cytokine production. However, the capsular anti-inflammatory properties outweigh the inflammatory properties of these two components. Stimulation with encapsulated cryptococci downregulate the activity of macrophages, dendritic cells, and neutrophils. This is associated with reduced inflammatory cytokine and chemokine production. GXM has also been shown to stimulate production of the inflammatory cytokine IL-10 and decrease production of pro-inflammatory cytokines. The capsule is also capable of accelerating degradation of the complement component C3b, as well as apoptosis through upregulation of FasL on APCs such as macrophages. Although these mechanisms do not prevent immunological recognition, they effectively provide a larger window of opportunity to upregulate its own mechanisms to enhance long-term survival in the host.
App1
Secreted cryptococcal protein App1 inhibits phagocytosis through a complement-mediated mechanism, and deletion of App1 improves phagocytosis by alveolar macrophages. App1 blocks complement 3 and complement 2 receptors (CR3 and CR2), interfering with phagocytosis [4]
Modulation of the adaptive immune response
C. neosporans is also capable of altering the adaptive immune response, which is crucial for long-term persistence. C. neosporans expresses a variety of mechanisms for this purpose: T-cell inhibition and apoptosis, promotion of a nonprotective Th2 response, interference with dendritic cell development, inhibition of protective Th17 response, induction of immune tolerance, and interference with antibody production. High virulence in certain strains has been linked with their ability to strongly induce IL-10, inhibiting development of Th1 responses and promoting tolerogenic responses.
Epidemiology
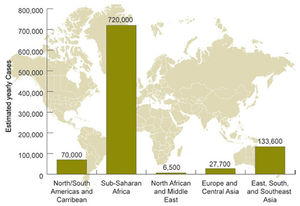
A close correlation between the occurrence of C. neoformans and pigeon droppings has been shown [5]. C. neoformans var. grubii has a worldwide distribution with the most cases being reported in the United States and Australia, while C. neoformans var. neoformans is is mostly restricted to France, Italy, and Denmark [6]. Among immmunocompetent individuals, groups at risk are the elderly and those using corticosteroids [5]. In the United States, only 25% of infections are reported as symptomatic among immunocompetent individuals [4]. Among HIV/AIDS patients, it is estimated that approximately 957,900 cases occur each year, resulting in nearly 625,000 deaths [3]. Worldwide, 7-10% of patients with AIDS are affected [3]. AIDS-associated cryptococcosis accounts for 50% of cryptococcal infections reported worldwide, usually occurring in HIV patients when their CD4 lymphocyte count falls below 200/mm3. In sub-Saharan Africa, the mortality rate is estimated to be between 50-70% . In developed countries such as the United States where therapy is available, the mortality rate is as low as 12% [3].
Clinical features
Cryptococcus neoformans most often infects the lungs or the central nervous system, but it can also affect other parts of the body as well [7]. Symptoms are dependent on which area of the body is infected. In immunocompetent individuals, infections are usually asymptomatic and do not spread past the lungs.
Lungs
C. neoformans infection in the lung causes typical pneumonia-like symptoms, including shortness of breath, coughing, and fever.
Central nervous system
Cryptococcal meningitis is the result of an infection spreading past the blood-brain barrier, allowing access to the central nervous system. Cryptococcal meningitis is a serious illness, commonly associated with swelling and irritation of the brain and spinal cord. Neurological symptoms do not typically occur until days or weeks after infection and include fever, hallucinations, headache, confusion, nausea and vomiting, light sensitivity, stiff neck, and blurred vision [8].
Diagnosis
Diagnostic features are difficult to distinguish, as visible findings such as skin, pulmonary, or bone lesions seen on X-ray are also characteristic of histoplasmosis, toxoplasmosis, and tuberculosis. A CT scan or MRI of the brain may show areas of possible infection, although many other diseases present similar findings as well. Serological testing of spinal fluid may be used if the patient is suspected to have an infection. Definitive diagnosis depends on isolation of the fungus from the patient's tissue or bodily fluids or identification from tissue biopsy samples. Further analysis, such as PCR, may distinguish whether the infection is caused by C. neoformans or C. gattii [9].
If the infection progresses to meningitis, more diagnostic features are present. These include faster heart rate, fever, mental state changes, and stiff neck. If meningitis is suspected, a lumbar puncture is an important test for removal of a sample of cerebrospinal fluid for further inspection.
Treatment
In healthy individuals, treatment is usually not necessary. Still, it is recommended to receive check-ups for up to monitor any spreading of the infection. If there are lung lesions or the disease spreads, antifungals such as fluconazole, itraconazole, and voriconazole may be prescribed to clear the infection. Patients with severe lung infections or infections of the central nervous system are recommended to take amphotericin B in combination with flucytosine. In some cases, surgery may be necessary to remove fungal growths (cryptococcomonas) [7].
Prevention
As Cryptococcus neosporans is spread throughout the environment via bird droppings, it is difficult to establish recommendations for prevention. Immunocompromised individuals should avoid areas known to be contaminated with bird droppings and should avoid contact with birds.
Host immune response
Despite interaction with contaminated areas, most immunocompetent individuals are able to contain the fungus or maintain the yeast in latent state. Besides barriers such as skin and nasal mucosa, a variety of innate factors are able to interfere with the establishment of infection. Human serum and saliva has been shown to exhibit anticryptococcal activity [10]. However, the complement system and phagocytes are the main responses to infection.
Complement
The complement system leads to inflammation and recruitment of phagocytic effector cells as well as the formation of the membrane attack complex. Animal model systems and human patients have demonstrated the importance of the complement system [10]. Guinea pigs treated with cobra venom to deplete complement components had shorter survival time, and mice deficient in C5 are more susceptible to infection and succumb three times quicker than C5 positive mice [11]. The survival time of C4-deficient guinea pigs is similar to that of normal guinea pigs, suggesting that the C4-independent alternative pathway is a viable response to infection. The lectin pathway of the complement system is dependent on capsulation of Cryptococcus neoformans as the capsule inhibits the binding of mannan-binding lectin.
Phagocytic effector cells
As the first line of defense against infection in the bloodstream, the complement system achieves certain preparations for further response through opsonization and phagocytic effector cell recruitment. In humans, neutrophils and macrophages are recruited for phagocytosis. Phagocytosis is triggered through direct contact with conserved pathogenic structures such as components of the cryptococcal capsule or through indirect recognition of antibody-opsonized cells. Dendritic cells function as major antigen-presenting cells and also initiate cell-mediated immunity. T-cell response by dendritic cells is much more efficient than by alveolar or peritoneal macrophages. At the site of infection, the number of neutrophil granulocytes increases as well. Oxidative bursts by neutrophils also act to remove the infection, although these bursts also damage surrounding host cells. Considering the still low number of neutrophils present at the site of infection, it is possible that neutrophils provide more of a regulatory rather than antimicrobial role. Recent studies, however, have suggested that macrophages may actually be harmful in infections as C. neoformans has developed several exploitative mechanisms to utilize macrophages [12].
Cell-mediated immunity
Individuals with HIV, leukemia, lymphoma, or on extensive corticosteroid treatment all belong to the group at highest risk for symptomatic cryptococcal infections [3]. The common feature of each of these groups is a defect in cell-mediated immunity, demonstrating its significance in combating the infection. Cell-mediated immunity refers to the killing of pathogens through direct killing via cytotoxic effects or indirect killing via NK cells and T lymphocytes. NK, CD4+, and CD8+ cells all act against the infection, and the secretion of granulysin and perforin are effective in cryptococcal permeabilization and lysis. In HIV patients, the low CD4+ count prevents secretion of these molecules.
References
1. Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, et al. 2010. Cryptococcal Cell Morphology Affects Host Cell Interactions and Pathogenicity. PLoS Pathog
2. Karkowska-Kuleta, J, Rapala-Kozik, M, Kozik, A. 2009. Fungi pathogenic to humans: Molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochimica Polonica
3. Cogliati M. 2013. Global Molecular Epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An Atlas of the Molecular Types. Scientifica
4. Olszewski MA, Zhang Y, Huffnagle GB. 2010. Mechanisms of Cryptococcal Virulence and Persistence. Future Microbiology
5. Lonian RD. 1965. The Effects of Steroids on the Virulence of Cryptococcus neoformans.Oklahoma State University.
6. The University of Adelaide. 'Cryptococcus neoformans.' Mycology Online.
7. http://www.cdc.gov/fungal/diseases/cryptococcosis-neoformans/
8. http://www.nytimes.com/health/guides/disease/cryptococcosis/overview.html
9. http://www.emedicinehealth.com/cryptococcosis/article_em.htm
10. http://ec.asm.org/content/9/6/835.full
11. Rhodes JC, Wicker LS, Urba WJ. 1980. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect. Immun.
12. Kechichian TB, Shea J, Del Poeta M. 2007. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect. Immun.
Created by Chris Vu, student of Tyrell Conway at the University of Oklahoma.
