Rio Tinto (Spain): Difference between revisions
| Line 57: | Line 57: | ||
===Protist research=== | ===Protist research=== | ||
==== From Genes to Genomes: Beyond Biodiversity in Spain's Rio Tinto==== | |||
Within the experiment scientists wanted to study how protist acidophiles (whom are very closely related to neutrophiles) were able to adapt to acidic conditions. They have isolated phylogenetically diverse protists to compare ion transporting ATPases between acidophiles and neutrophiles. It was hypothesized that special properties of ion transporters are what is responsible for protists to survive in the Rio Tinto. It was later concluded that there are still many undiscovered lineages within the system that allow for the survival of the protists. [6] | |||
==References== | ==References== | ||
Revision as of 06:22, 28 April 2010
Introduction
The Rio Tinto is a river in Southwestern Spain. It is a total of 100km long and flows from the city of Pena de Hierro out to the Atlantic Ocean in Huela. The waters of the Rio Tinto flow red due to a high concentration of ferric iron. The river is known as an extreme environment due to its low pH and high concentrations of heavy metals. The region has been an important copper mining area for the last 5000 years. It was originally thought that the low pH was caused by the copper mining, but it was later discovered that the low pH is a result of microbial activity and not of the anthropogenic impacts. The microbial eukaryotic communities show a stronger correlation with the concentration of heavy metals then with the pH of the water. What makes this acidic water system so unique is the fact that its primary contributor of biomass comes from eukaryotic micro organisms. It is estimated that up to 65% of total biomass within the system comes from these eukaryotic communities. The location of the primary biomass occurs on the river bends where a dense compact Stream biofilm forms. These biofilms are primarily composed of fungi and algae. This is unique because most acidic waters with high metal concentrations are toxic to eukaryotes and limit both growth and biodiversity.
Physical environment
The Rio Tinto is known for its extreme environmental conditions of low pH and high concentrations of heavy metals. On average the pH of the water is around 2, but may very through out the water system. Some areas measured as low as a pH 1.1 while other areas have recorded pH levels as high as 3. It has been observed that the pH tends to be lower in beginning of the river and higher as the river nears the mouth. There are three primary heavy metals found in the water column, they are iron, copper, and zinc. Their averages through out the year can range from 0.4g/L-20.3g/L for Iron, 0.02g/L-0.7g/L for copper, and 0.02g/L-0.56g/L for zinc. The concentrations of heavy metals within the water column have been found to vary seasonally. In the summer months of June through September the heavy metal concentrations seem to be the highest. It is thought that this occurs due to that time of year being the dry season and having warmer temperatures. This results in lower water levels due to less rain and increased evaporation. These two conditions concentrate the heavy metals within the water system since less water is entering and more is leaving before ever reaching the mouth at the Atlantic. The average water temperatures range from 25*C in the summer to 15*C in the winter. As with most aquatic ecosystems the dissolved oxygen levels also very seasonally with the winter months containing higher concentrations of dissolved oxygen when compared to the warmer summer months.
Biological interactions
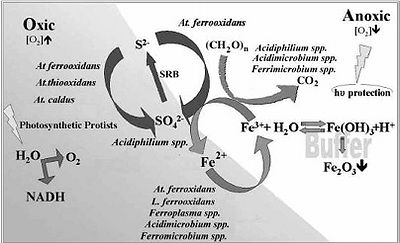
The most important biological interaction within the Rio Tinto is the decrease in pH due to the oxidation of sulfur by acidic chemolithotrophic organisms. This effects everything within the aquatic ecosystem. Its so extreme that the average microbe cant even sustain life there. Everything within the ecosystem seems to revolve around the Iron and Sulfur cycles within the system. Iron oxidizing prokaryotes and iron reducers are capable of functioning in both anaerobic and aerobic conditions. The Ferric Iron produced is a limiting factor placed upon the Sulfur cycle. Below is a diagram to better show how Iron and Sulfur cycles are tied together, along with how the microbial populations work together to complete the cycles.
Microbial processes
The microbial processes that define this environment are the oxidation of iron and sulfur. Sulfides are the main substrate of choice for the acidic chemolithotrophic organisms present in the Rio Tinto. Sulfides first undergo oxidation threw the polysulfide mechanism which will then produce elemental sulfur. At this state the microbes are able to further oxidize the sulfur into sulfuric acid. This is where the Rio Tinto's high pH levels stems from. The equations below can better help to show what is truly going on.
1) Fe3+ + 3H2O ⇔ Fe(OH)3 + 3H+
2) 8MS + 8Fe3+ + 8H+ → 8M2+ + 4H2Sn + 8Fe2+
3) 4H2Sn + 8Fe3+ → S08 + 8Fe2+ + 8H+
4) S08 + 12O2 + 8H2O → 8SO2−4 + 16H+
The remaining insoluble elements such as pyrite, tungstenite and molybdenite may oxidize into the water column only by means of the thiosulfate mechanism which needs ferric iron. Example shown below.
5) FeS2 + 6Fe3+ + 3H2O → 7Fe2+ + S2O2−3 + 6H+
6) S2O2−3 + 8Fe3+ + 5H2O → 2SO2−4 + 8Fe2+ + 10H+
Key Microorganisms and Examples
Chemolithotrophs
These types of microbes oxidize inorganic compounds to obtain energy. Chemolithotrophs in the Rio Tinto are known as extremophiles as they are able to not only survive but also thrive in conditions that would normally kill or harm other microbes. To survive in the Rio Tinto these chemolithotrophs would need adaptations that would allow them to deal with the extreme pH levels along with the strong concentrations of heavy metals. The types of chemolithotrophs found at the Rio Tinto may be classified as sulfur oxidizing and iron oxidizing. The primary sulfur oxidizing microbe found in the Rio Tinto is classified as Acidithiobacillus ferroxidans. There were also 3 other microbes found although they are not considered significant in numbers. Some others were in the family of Acidithiobacillus genus and At. thiooxidans. There have been many Iron oxidizing microbes found within the Rio Tinto. The two most important iron oxidizing microbes have been determined using specific fluorescent probes. The results showed that L.ferrooxidans and At. ferrooxidans are the most important. Other microbes were found and identified as Leptospirillum ferrooxidans, Acidithiobacillus ferrooxidans, and Ferroplasma spp.
Photosynthetic Primary Producers
These microbes are able to produce their own food by means of extracting energy from sunlight. With this energy they are then able to fix carbon and perform other required tasks for survival. The most important Photosynthetic Primary Producers in the Rio Tinto is Acidophilic Algae. These microbes also require special adaptations to withstand low pH and strong concentrations of heavy metals. As stated previously acidophilic algae accounts for 65% of biomass within the Rio Tinto ecosystem. The organisms identified were Bacillariophyceae (Diatoms), Chlorophyta (Chlamydomonas, Klebsormidium and Zignema), Euglenozoa (Euglena), and Rhodophyta (Galdieria)
Consumers
The primary consumers within the Rio Tinto are Eukaryotic Heterotrophic Protists. This means that they are organisms with a cell nucleus(eukaryote), and use organic carbon for growth since they are not able to produce their own food(Heterotroph). The major group of consumers within the Rio Tinto are Eukaryotic Hetertrophic protists. Some examples of these organisms are amoebae of the class Lobosea(phylum Rhizopoda), flagellates, some individuals from the class of Heliozoa (phylum Actinopoda) and ciliates(phylum Ciliophora) which are mainly associated with biofilms.
Decomposers
These microbes are heterotrophic and rely on dead organisms to obtain carbon and other nutrients to sustain themselves. In the Rio Tinto ecosystem most decomposers found are either fungi or yeasts. The most important acidic fungi group found belonged to the Dematiaceous group. This also included members of Bahusakala, Heteroconium, Phoma and Scytalidium genus. So far studies have also showed sixteen different forms of Penicillium spp. There have also been seven different genras of yeast found within the aquatic system, they are Candida, Cryptococcus, Holtermannia, Leucosporidium, Tremella, Rhodotorula and Hansenula.
Current Research
Mars research
Photoreduction fuels biogeochemical cycling of iron in Spain's acid rivers
The purpose of this research was to analyze the photoreduction properties of Fe(III) for the Rio Tinto ecosystem. This will allow a better understanding of if this process is capable of supplying enough Fe(II) to be oxidized by bacteria, thus releasing enough free energy to support biological processes on mars. The conclusion is that this process could possibly play an important part on mars or other planetary systems.[4]
Mars Astrobiology Research and Technology Experiment (MARTE)
This experiment entailed testing recently developed equipment for landing and drilling into the surface of Mars in search of subsurface life. The Rio Tinto site was chosen due to having similar characteristics to Mars. The experiment was done with robotics that drilled deep into the surface of the Rio Tinto(up to 6 meters deep). All drillings were remotely performed by scientists off site. The conclusion was that it is entirely possible for a procedure similar to this one to be successful on the surface of mars.[5]
Protist research
From Genes to Genomes: Beyond Biodiversity in Spain's Rio Tinto
Within the experiment scientists wanted to study how protist acidophiles (whom are very closely related to neutrophiles) were able to adapt to acidic conditions. They have isolated phylogenetically diverse protists to compare ion transporting ATPases between acidophiles and neutrophiles. It was hypothesized that special properties of ion transporters are what is responsible for protists to survive in the Rio Tinto. It was later concluded that there are still many undiscovered lineages within the system that allow for the survival of the protists. [6]
References
[1] Amils R., E. Gonz´alez-Toril, D. Fern´andez-Remolar, F. G´omez, N. Rodr´?guez, and C. Dur´an. "Interaction of the Sulfur and Iron Cycles in the Tinto River Ecosystem." Environmental Science & Bio/Technology (2002): 299-309. Geobase. Web. 11 Apr. 2010
[2] Aquilera, Angeles, Susanna C. Manrubia, Felipe Gomez, Nuria Rodriguez, and Ricardo Amils. "Eukaryotic Community Distribution and Its Relationship to Water Physicochemical Parameters in an Extreme Acidic Environment, Río Tinto (Southwestern Spain)dagger." Applied and Environmental Microbiology 72.8 (2006): 5325-330. American Society for Microbiology. Web. 11 Apr. 2010.
[3] Baker, Brett J., Gene W. Tyson, Lindsey Goosherst, and Jillian F. Banfield. "Insights into the Diversity of Eukaryotes in Acid Mine Drainage Biofilm Communities." American and Environmental Microbiology 75.7 (2009): 2192-199. American Society For Microbiology. Web. 11 Apr. 2010.
[4] Nimick, David A., Christopher H. Gammons, Stephen R. Parker, Dean M. Snyder, Blaine R. McCleskey, Ricardo Amils, and Simon R. Poulson. "Photoreduction Fuels Biogeochemical Cycling of Iron in Spain's Acid Rivers." Photoreduction Fuels Biogeochemical Cycling of Iron in Spain's Acid Rivers2 252.3-4 (2008): 202-13. Science Direct. Web. 11 Apr. 2010.
[5] Stoker, Carol R., Howard N. Cannon, Stephen E. Dunagan, Lawrence G. Lemke, and Brian J. Glass. "The 2005 MARTE Robotic Drilling Experiment in Río Tinto, Spain: Objectives, Approach, and Results of a Simulated Mission to Search for Life in the Martian Subsurface." Astrobiology 8.5 (2005). Scopas. Web. 11 Apr. 2010.
[6] Zettler, Linda A., Mark A. Messerli, Abby D. Laatsch, Peter J. Smith, and Mitchell L. Sogin. "From Genes to Genomes: Beyond Biodiversity in Spain's Rio Tinto." Marine Biological Laboratory 204 (2003): 205-09. Partner Access. Web. 11 Apr. 2010
[7] fotonatura.org. picture 1 by Armando Aguilera Pérez
Edited by student of Angela Kent at the University of Illinois at Urbana-Champaign.