Membrane proteins as mechanisms of immune system evasion in Trypanosoma brucei: Difference between revisions
Stamatoiua (talk | contribs) No edit summary |
Stamatoiua (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
[[Image:Trypanosoma-brucei.jpg|thumb|300px|right|<b>Figure 1.</b> Blood smear with <i>Trypanosoma brucei</i> from the Center for Disease Control and Prevention.]] | [[Image:Trypanosoma-brucei.jpg|thumb|300px|right|<b>Figure 1.</b> Blood smear with <i>Trypanosoma brucei</i> from the Center for Disease Control and Prevention.]] | ||
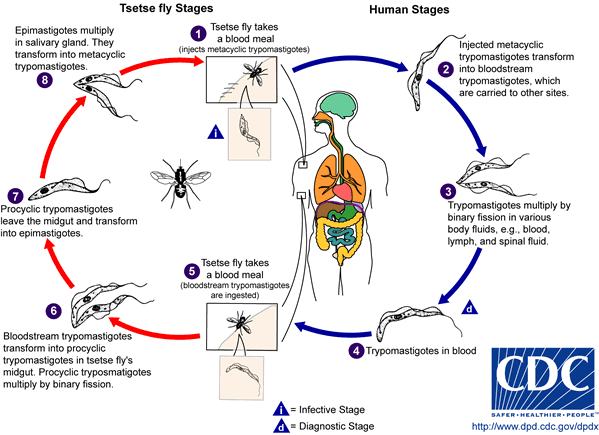
<br>Of all | <br>Of all defined parasitic human pathogens, the transmitter of Trypanosomiasis (commonly known as African Sleeping Sickness) has a truly fascinating relationship to its hosts. As heteroxenous organisms, Trypanosoma parasites require more than one host to successfully complete their life cycles. Specifically, these flagellate protozoa are unicellular and carry out part of their growth inside the gut of the tse tse fly prior to transmission into the human bloodstream. The mechanisms through which Trypanosoma species evade the human immune system are complex and not completely defined. Much research is concerned with studying the exact modes these unique organisms have for escaping antibody detection and wreaking havoc on their hosts’ health. Perhaps the most important technique Trypanosoma species have is the ability to manipulate their membrane receptor proteins at a more rapid speed than human immune systems can assemble appropriate antibodies. Inadequacy of current treatments has spurred on research to describe this phenomenon in greater detail and discover more effective means of understanding the many facets of African sleeping sickness. | ||
<br><b>Subscript:</b> H<sub>2</sub>O | <br><b>Subscript:</b> H<sub>2</sub>O | ||
<br><b>Superscript:</b> Fe<sup>3+</sup> | <br><b>Superscript:</b> Fe<sup>3+</sup> | ||
Revision as of 01:59, 23 April 2013
Introduction
Of all defined parasitic human pathogens, the transmitter of Trypanosomiasis (commonly known as African Sleeping Sickness) has a truly fascinating relationship to its hosts. As heteroxenous organisms, Trypanosoma parasites require more than one host to successfully complete their life cycles. Specifically, these flagellate protozoa are unicellular and carry out part of their growth inside the gut of the tse tse fly prior to transmission into the human bloodstream. The mechanisms through which Trypanosoma species evade the human immune system are complex and not completely defined. Much research is concerned with studying the exact modes these unique organisms have for escaping antibody detection and wreaking havoc on their hosts’ health. Perhaps the most important technique Trypanosoma species have is the ability to manipulate their membrane receptor proteins at a more rapid speed than human immune systems can assemble appropriate antibodies. Inadequacy of current treatments has spurred on research to describe this phenomenon in greater detail and discover more effective means of understanding the many facets of African sleeping sickness.
Subscript: H2O
Superscript: Fe3+
Disease Origins and Distribution
Include some current research in each topic, with at least one figure showing data.
Mechanism of Infection
Include some current research in each topic, with at least one figure showing data.
Symptoms, Diagnosis, and Treatment
some stuff
Protein Cycling
talk about proteins
Drug Interactions
drugs
Future Research
further description of mechanism of action (current data)
Conclusion
Overall paper length should be 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.