Chlorhexidine: Difference between revisions
Mserrano6633 (talk | contribs) |
Mserrano6633 (talk | contribs) |
||
| (63 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
<br> Chlorhexidine is used as a prescription mouthwash to fight gingivitis and periodontists. Chlorhexidine is the most thoroughly researched antimicrobial agent used in dentistry in terms of ability to control cariogenic activity. It is effective in reducing plaque and gingivitis in teeth and gums but can causes an alteration in taste and some discoloration of teeth. Originally Listerine was the most commonly used mouthwash to prevent plaque buildup, but research into other types of mouthwashes lead to the use of chlorhexidine as a form of treating periodontist and gingivitis, as it was found to be the most effective in reducing plaque and gingivitis.<br> | <br> Chlorhexidine is used as a prescription mouthwash to fight [http://en.wikipedia.org/wiki/Gingivitis gingivitis] and [http://en.wikipedia.org/wiki/Periodontal_pathology periodontists]<sup>[[#References | [4]]]</sup>. Chlorhexidine is the most thoroughly researched antimicrobial agent used in dentistry in terms of ability to control [http://en.wikipedia.org/wiki/Dental_caries cariogenic activity]<sup>[[#References | [10]]]</sup>. It is effective in reducing plaque and gingivitis in teeth and gums but can causes an alteration in taste and some discoloration of teeth<sup>[[#References | [4]]]</sup>. Originally Listerine was the most commonly used mouthwash to prevent plaque buildup, but research into other types of mouthwashes lead to the use of chlorhexidine as a form of treating periodontist and gingivitis, as it was found to be the most effective in reducing plaque and gingivitis<sup>[[#References | [1]]]</sup>.<br> | ||
<br> Chlorhexidine works by modifying the permiability of the cell membrane and either stops bacteria from reproducing or kills them<sup>[[#References | [10]]]</sup>. Though chlorhexidine mouthwash is the most studied there are other forms it can be used in such as varnishes and gel, each having its own benefits<sup>[[#References | [10]]]</sup>. There are also alternatives to chlorhexidine which have similar effects on oral bacteria but without negative side-effects<sup>[[#References | [4]]]</sup><sup>[[#References | [7]]]</sup>. | |||
[[File:Chlorhexidine ball-and-stick.png|300px|thumb|Figure 1.Ball and stick model of Chlorhexidine [http://commons.wikimedia.org/wiki/File%3AChlorhexidine_ball-and-stick.png By MarinaVladivostok (Own work) [CC0], via Wikimedia Commons]]] | |||
==Mechanism of Action== | |||
[[File:Intraorally developed biofilm.jpg|200px|thumb|left|Figure 2. Biofilm treated with chlorhexidine on a tooth. [http://commons.wikimedia.org/wiki/File%3AIntraorally_developed_biofilm.jpg By Ronald Ordinola Zapata (Own work) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons]]] | |||
<br> Chlorhexidine is an antimicrobial agent that alters the composition of microorganisms<sup>[[#References | [10]]]</sup>. Chlorhexidine reduces the proportions of some microorganisms which are especially sensitive to this substance<sup>[[#References | [10]]]</sup>. Chlorhexidine works by altering the metabolic activity of bacteria<sup>[[#References | [10]]]</sup>. In lower concentrations Chlorhexidine is [http://en.wikipedia.org/wiki/Bacteriostatic_agent bacteriostatic], essentially preventing bacteria from reproducing, and prompts both changes in functioning of the cell membrane as well as leakage of the intracellular components<sup>[[#References | [10]]]</sup>. In high concentrations Chlorhexidine acts as a bactericide causing precipitation of the cellular content and inhibits the action of the [http://en.wikipedia.org/wiki/Glycosyltransferase glycosyltransferase enzyme] which is responsible for the accumulation of bacteria on the dental surface and has effects on sugar transport and acid production in oral bacteria<sup>[[#References | [10]]]</sup>. <br> | |||
<br>In one study the effect of chlorhexidine on transport of methyl-β-D-thiogalactoside (TMG) was tested<sup>[[#References | [8]]]</sup>. They tested the effects of different concentrations of chlorhexidine and measured the levels of TMG in <i>Escherichia coli</i> at ten minutes<sup>[[#References | [8]]]</sup>. It was found that as the concentration of chlorhexidine increased so did the inhibition of TMG and the viability of the cells decreased<sup>[[#References | [8]]]</sup>. Never-the-less it was found that 50% of cells survived even after 24 hours of contact with chlorhexidine so the inhibition of transport was not lethal but it does show that chlorhexidine effects the permiability of the membrane<sup>[[#References | [8]]]</sup>. <br> | |||
==Effects of Different Forms of Chlorhexidine== | |||
<br>Chlorhexidine can be applied in varies forms. Some of which were compared were: mouthwash (0.12% and 0.2%), gels (1%), and varnishes (1%, 10%, 20%, and 35%)<sup>[[#References | [7]]]</sup>. <br> | |||
== | ===Mouthwash=== | ||
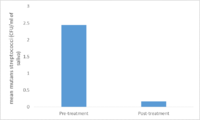
[[File:chlorhexidine.png|200px|thumb|left|Figure 3. A comparison of <i>Streptococci mutans count</i> (CFU/ml of saliva) in test groups as | |||
compared to the control group<sup>[[#References | [7]]]</sup>.There was a significant decrease in <i>Streptococci mutans count</i> count in the test group compared to the control group<sup>[[#References | [7]]]</sup>. Image created by Martha Serrano]] | |||
<br> Chlorhexidine | <br>Rinsing with 0.12% and 0.2% Chlorhexidine solutions showed a significant decrease in [http://en.wikipedia.org/wiki/Streptococcus_mutans <i>Streptococci mutans</i>] after 24 hours<sup>[[#References | [10]]]</sup>. On the other hand rinsing with Chlorhexidine mouthwash solution did not produce any long term effects on <i>Streptococci mutans</i><sup>[[#References | [10]]]</sup>. Only one of the solutions, 0.12% Chlorhexidine, had a lasting effect on salivary <i>Streptococci mutans</i> levels after six weeks of treatment. Treatments using 0.12% Chlorhexidine solutions for 1 or 2 weeks, with 1 or 2 rinsings each day, did not produce any significant long-term reduction in salivary <i>Streptococci mutans</i><sup>[[#References | [10]]]</sup>. Though in another study done with 0.12% chlorhexidine it was found that rinsing twice a day for two weeks significantly decreased <i>Streptococci mutans</i><sup>[[#References | [7]]]</sup>. Neither did increasing the Chlorhexidine concentration to 0.2% and the treatment period to 4 weeks. The randomized and controlled studies showed that rinsing with a Chlorhexidine mouthwash solution had no long-term effect on salivary <i>Streptococci mutans</i><sup>[[#References | [10]]]</sup>. <br> | ||
===Gel=== | |||
<br>The main advantage of the gel over mouthwash is that it prolongs the [http://en.wikipedia.org/wiki/Bioavailability bioavailability] of chlorhexidine <sup>[[#References | [5]]]</sup>. A review on the literature of chlorhexidine gel at 0.2%, 0.5%, 1% , and 5% were studied<sup>[[#References | [10]]]</sup>. Of these gels 0.2%, 0.5%, and 1% were applied with a toothbrush, while 1% and 5% gels are applied through professional prophylaxis and trays<sup>[[#References | [9]]]</sup>. Chlorhexidine gel significantly decreased <i>Streptococci mutans</i> levels for a period of 4-26 weeks after intense treatment which included 3-4 daily applications for 2 days or through daily applications for periods of 10 and 14 days<sup>[[#References | [10]]]</sup>. In another study conducted comparing the effectiveness of 0.12% chlorhexidine in mouthwash and 0.2% chlorhexidine in gel it was found that the gel had a lower incidence of [http://en.wikipedia.org/wiki/Alveolar_osteitis alveolar osteitis] than the mouthwash, 7.5% versus 25%<sup>[[#References | [5]]]</sup>.<br> | |||
===Varnish=== | |||
<br>Chlorhexidine varnish is often used after [http://en.wikipedia.org/wiki/Scaling_and_root_planing scaling and root planning] to treat chronic periodontists as it last longer than mouthwash<sup>[[#References | [2]]]</sup>. Chlorhexidine varnish displayed large variations in the level and length of decreased <i>Streptococci mutans</i><sup>[[#References | [10]]]</sup>. Depending on the subject the decrease in <i>Streptococci mutans</i> could last for 3 days to 2 weeks<sup>[[#References | [10]]]</sup>. Also the greater the concentration the greater the decease level in <i>Streptococci mutans</i>.In another study varnish was also found to decrease [http://en.wikipedia.org/wiki/Periodontal_probe probing depth], reducing pockets by between 0.70 and 1.37 mm <sup>[[#References | [2]]]</sup><br> | |||
== | ===Conclusion=== | ||
<br>Chlorhexidine varnish and gel were the most effective out of the three types of chlorhexidine treatments studied<sup>[[#References | [5]]]</sup><sup>[[#References | [10]]]</sup>. Out of the two chlorhexidine varnish was found to be more effective than the gel<sup>[[#References | [10]]]</sup>.<br> | |||
<br>Chlorhexidine varnish and gel were the most effective out of the three types of chlorhexidine studied. Out of the two chlorhexidine varnish was found to be more effective than the gel.<br> | |||
<br> | <br> | ||
==Alternatives to Chlorhexidine== | ==Alternatives to Chlorhexidine== | ||
<br>Though chlorhexidine is effective | <br>Though chlorhexidine is an effective way of treating periodontal diseases it does have negative side effects namely bad odor, a slight staining in teeth, and a change in taste perception<sup>[[#References | [3]]]</sup><sup>[[#References | [4]]]</sup>.Chlorhexidine reduced the taste intensity of NaCl to 35 ± 5% and quinine HCl to 50 ± 8% of pretreatment strength<sup>[[#References | [3]]]</sup>. There was no effect on sucrose; the mean posttreatment intensity was 97 ± 5% of pretreatment but citric acid intensity increased to 139 ± 13%<sup>[[#References | [3]]]</sup>.There have studies that found alternatives which work just as well as chlorhexidine without the negative effects.<br> | ||
===Triphala mouthwash=== | |||
<br>Triphala is a combination of three different plant extracts used in a mouthwash to treat diseases because of its antimicrobial and antioxidant properties. Triphala was found to be as effective as 2% chlorhexidine in treating plaque | <br>[http://en.wikipedia.org/wiki/Triphala Triphala] is a combination of three different plant extracts ([http://en.wikipedia.org/wiki/Phyllanthus_emblica Amalaki], [http://en.wikipedia.org/wiki/Terminalia_bellirica Bibhitaki], and [http://en.wikipedia.org/wiki/Terminalia_chebula Haritaki]). used in a mouthwash to treat diseases because of its antimicrobial and antioxidant properties<sup>[[#References | [9]]]</sup>. Triphala was found to be as effective as 2% chlorhexidine in treating plaque<sup>[[#References | [9]]]</sup>. <br> | ||
< | |||
<br>The plant extract of Terminalia chebula was compared with chlorhexidine to see if it would be a good alternative to treat dental caries, gingivitis and stomatitis. There was a significant decrease in plaque in both the chlorhexidine group and Terminalia chebula. There was no significant difference between both group and both reduced plaque buildup and gingivitis. <br | ===Terminalia chebula=== | ||
<br>The plant extract of [http://en.wikipedia.org/wiki/Terminalia_chebula Terminalia chebula] was compared with chlorhexidine to see if it would be a good alternative to treat dental caries, gingivitis and stomatitis<sup>[[#References | [4]]]</sup>. There was a significant decrease in plaque in both the chlorhexidine group and Terminalia chebula<sup>[[#References | [4]]]</sup>. There was no significant difference between both group and both reduced plaque buildup and gingivitis<sup>[[#References | [4]]]</sup>. <br> | |||
==Further Reading== | |||
Though chlorhexidine has its benefits it might also cause heart disease. In a study conducted in 2013, they used mouthwash which contained chlorhexidine to suppress oral microbes to determine if it affects systemic nitrite levels and blood pressure in healthy individuals, as nitrite is found to be a precursor for systemic generation of vasodilatory nitric oxide, and external administration of nitrate reduces blood pressure in humans<sup>[[#References | [6]]]</sup>. | |||
==References== | ==References== | ||
Adams, D., and M. Addy. "Mouthrinses." Advances in Dental Research 8.2 (1994): 291. Web. | <sup>1</sup> [http://adr.sagepub.com/content/8/2/291 Adams, D., and M. Addy. "Mouthrinses." Advances in Dental Research 8.2 (1994): 291. Web.] | ||
<sup>2</sup>[http://www.joponline.org/doi/abs/10.1902/jop.2006.050220?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed Cosyn, J., et al. "Clinical Benefits of Subgingival Chlorhexidine Varnish Application as an Adjunct to Same-Day Full-Mouth Root Planing: A Pilot Study." Journal of periodontology 77.6 (2006): 1074-9. Web.] | |||
<sup>3</sup>[http://www.sciencedirect.com/science/article/pii/S0031938401005583 Frank, Marion E., Janneane F. Gent, and Thomas P. Hettinger. "Effects of Chlorhexidine on Human Taste Perception." Physiology & Behavior 74.1 (2001): 85-99. Web.] | |||
<sup>4</sup>[http://onlinelibrary.wiley.com/doi/10.1002/ptr.5075/abstract Gupta, Devanand, et al. "Effect of Terminalia Chebula Extract and Chlorhexidine on Salivary pH and Periodontal Health: 2 Weeks Randomized Control Trial." Phytotherapy Research 28.7 (2014): 992-8. Web.] | |||
<sup>5</sup>[http://www.sciencedirect.com/science/article/pii/S0278239107014814 Hita-Iglesias, P., et al. "Effectiveness of Chlorhexidine Gel Versus Chlorhexidine Rinse in Reducing Alveolar Osteitis in Mandibular Third Molar Surgery." JOURNAL OF ORAL AND MAXILLOFACIAL SURGERY 66.3 (2008): 441-5. Web.] | |||
<sup>6</sup>[http://www.sciencedirect.com/science/article/pii/S0891584912018229 Kapil, Vikas, et al. "Physiological Role for Nitrate-Reducing Oral Bacteria in Blood Pressure Control." Free Radical Biology and Medicine 55 (2013): 93-100.] | |||
<sup>7</sup>[http://ry6af4uu9w.search.serialssolutions.com/?ctx_ver=Z39.88-2004&ctx_enc=info%3Aofi%2Fenc%3AUTF-8&rfr_id=info:sid/summon.serialssolutions.com&rft_val_fmt=info:ofi/fmt:kev:mtx:journal&rft.genre=article&rft.atitle=Comparative+evaluation+of+efficacy+of+sodium+fluoride%2C+chlorhexidine+and+triclosan+mouth+rinses+in+reducing+the+mutans+streptococci+count+in+saliva+%3A+an+in+vivo+study&rft.jtitle=Journal+of+the+Indian+Society+of+Pedodontics+and+Preventive+Dentistry&rft.au=Kulkarni%2C+V+V&rft.au=Damle%2C+S+G&rft.date=2003-09-01&rft.issn=0970-4388&rft.volume=21&rft.issue=3&rft.spage=98&rft_id=info:pmid/14703215&rft.externalDocID=14703215¶mdict=en-US Kulkarni, V. V., and S. G. Damle. "Comparative Evaluation of Efficacy of Sodium Fluoride, Chlorhexidine and Triclosan Mouth Rinses in Reducing the Mutans Streptococci Count in Saliva : An in Vivo Study." Journal of the Indian Society of Pedodontics and Preventive Dentistry 21.3 (2003): 98. Web.] | |||
<sup>8</sup>[http://femsle.oxfordjournals.org/content/100/1-3/211.long Kuyyakanond, T., and LB Quesnel. "The Mechanism of Action of Chlorhexidine." FEMS microbiology letters 100.1-3 (1992): 211-5. Web.] | |||
Naiktari, RS, et al. "A Randomized Clinical Trial to Evaluate and Compare the Efficacy of Triphala Mouthwash with 0.2% Chlorhexidine in Hospitalized Patients with Periodontal Diseases." JOURNAL OF PERIODONTAL AND IMPLANT SCIENCE 44.3 (2014): 134-40. Web. | <sup>9</sup>[http://ry6af4uu9w.search.serialssolutions.com/?ctx_ver=Z39.88-2004&ctx_enc=info%3Aofi%2Fenc%3AUTF-8&rfr_id=info:sid/summon.serialssolutions.com&rft_val_fmt=info:ofi/fmt:kev:mtx:journal&rft.genre=article&rft.atitle=A+randomized+clinical+trial+to+evaluate+and+compare+the+efficacy+of+triphala+mouthwash+with+0.2%25+chlorhexidine+in+hospitalized+patients+with+periodontal+diseases&rft.jtitle=Journal+of+Periodontal+%26+Implant+Science&rft.au=Naiktari%2C+Ritam+S&rft.au=Gaonkar%2C+Pratima&rft.au=Gurav%2C+Abhijit+N&rft.au=Khiste%2C+Sujeet+V&rft.date=2014&rft.issn=2093-2278&rft.eissn=2093-2286&rft.volume=44&rft.issue=3&rft.spage=134&rft_id=info:doi/10.5051%2Fjpis.2014.44.3.134&rft.externalDBID=n%2Fa&rft.externalDocID=10_5051_jpis_2014_44_3_134¶mdict=en-US Naiktari, RS, et al. "A Randomized Clinical Trial to Evaluate and Compare the Efficacy of Triphala Mouthwash with 0.2% Chlorhexidine in Hospitalized Patients with Periodontal Diseases." JOURNAL OF PERIODONTAL AND IMPLANT SCIENCE 44.3 (2014): 134-40. Web.] | ||
Ribeiro, Luciana Gazaniga Maia, Lina Naomi Hashizume, and Marisa Maltz. "The Effect of Different Formulations of Chlorhexidine in Reducing Levels of Mutans Streptococci in the Oral Cavity: A Systematic Review of the Literature." Journal of dentistry 35.5 (2007): 359-70. Web. | <sup>10</sup>[http://www.sciencedirect.com/science/article/pii/S0300571207000292 Ribeiro, Luciana Gazaniga Maia, Lina Naomi Hashizume, and Marisa Maltz. "The Effect of Different Formulations of Chlorhexidine in Reducing Levels of Mutans Streptococci in the Oral Cavity: A Systematic Review of the Literature." Journal of dentistry 35.5 (2007): 359-70. Web.] | ||
<!--Do not remove this line--> | <!--Do not remove this line--> | ||
Edited by (Martha Serrano), a student of [http://www.jsd.claremont.edu/faculty/profile.asp?FacultyID=254 Nora Sullivan] in BIOL168L (Microbiology) in [http://www.jsd.claremont.edu/ The Keck Science Department of the Claremont Colleges] Spring | Edited by (Martha Serrano), a student of [http://www.jsd.claremont.edu/faculty/profile.asp?FacultyID=254 Nora Sullivan] in BIOL168L (Microbiology) in [http://www.jsd.claremont.edu/ The Keck Science Department of the Claremont Colleges] Spring 2015. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Nora Sullivan at the Claremont Colleges]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Nora Sullivan at the Claremont Colleges]] | ||
Latest revision as of 20:53, 14 April 2015
Chlorhexidine is used as a prescription mouthwash to fight gingivitis and periodontists [4]. Chlorhexidine is the most thoroughly researched antimicrobial agent used in dentistry in terms of ability to control cariogenic activity [10]. It is effective in reducing plaque and gingivitis in teeth and gums but can causes an alteration in taste and some discoloration of teeth [4]. Originally Listerine was the most commonly used mouthwash to prevent plaque buildup, but research into other types of mouthwashes lead to the use of chlorhexidine as a form of treating periodontist and gingivitis, as it was found to be the most effective in reducing plaque and gingivitis [1].
Chlorhexidine works by modifying the permiability of the cell membrane and either stops bacteria from reproducing or kills them [10]. Though chlorhexidine mouthwash is the most studied there are other forms it can be used in such as varnishes and gel, each having its own benefits [10]. There are also alternatives to chlorhexidine which have similar effects on oral bacteria but without negative side-effects [4] [7].
Mechanism of Action
Chlorhexidine is an antimicrobial agent that alters the composition of microorganisms [10]. Chlorhexidine reduces the proportions of some microorganisms which are especially sensitive to this substance [10]. Chlorhexidine works by altering the metabolic activity of bacteria [10]. In lower concentrations Chlorhexidine is bacteriostatic, essentially preventing bacteria from reproducing, and prompts both changes in functioning of the cell membrane as well as leakage of the intracellular components [10]. In high concentrations Chlorhexidine acts as a bactericide causing precipitation of the cellular content and inhibits the action of the glycosyltransferase enzyme which is responsible for the accumulation of bacteria on the dental surface and has effects on sugar transport and acid production in oral bacteria [10].
In one study the effect of chlorhexidine on transport of methyl-β-D-thiogalactoside (TMG) was tested [8]. They tested the effects of different concentrations of chlorhexidine and measured the levels of TMG in Escherichia coli at ten minutes [8]. It was found that as the concentration of chlorhexidine increased so did the inhibition of TMG and the viability of the cells decreased [8]. Never-the-less it was found that 50% of cells survived even after 24 hours of contact with chlorhexidine so the inhibition of transport was not lethal but it does show that chlorhexidine effects the permiability of the membrane [8].
Effects of Different Forms of Chlorhexidine
Chlorhexidine can be applied in varies forms. Some of which were compared were: mouthwash (0.12% and 0.2%), gels (1%), and varnishes (1%, 10%, 20%, and 35%) [7].
Mouthwash
Rinsing with 0.12% and 0.2% Chlorhexidine solutions showed a significant decrease in Streptococci mutans after 24 hours [10]. On the other hand rinsing with Chlorhexidine mouthwash solution did not produce any long term effects on Streptococci mutans [10]. Only one of the solutions, 0.12% Chlorhexidine, had a lasting effect on salivary Streptococci mutans levels after six weeks of treatment. Treatments using 0.12% Chlorhexidine solutions for 1 or 2 weeks, with 1 or 2 rinsings each day, did not produce any significant long-term reduction in salivary Streptococci mutans [10]. Though in another study done with 0.12% chlorhexidine it was found that rinsing twice a day for two weeks significantly decreased Streptococci mutans [7]. Neither did increasing the Chlorhexidine concentration to 0.2% and the treatment period to 4 weeks. The randomized and controlled studies showed that rinsing with a Chlorhexidine mouthwash solution had no long-term effect on salivary Streptococci mutans [10].
Gel
The main advantage of the gel over mouthwash is that it prolongs the bioavailability of chlorhexidine [5]. A review on the literature of chlorhexidine gel at 0.2%, 0.5%, 1% , and 5% were studied [10]. Of these gels 0.2%, 0.5%, and 1% were applied with a toothbrush, while 1% and 5% gels are applied through professional prophylaxis and trays [9]. Chlorhexidine gel significantly decreased Streptococci mutans levels for a period of 4-26 weeks after intense treatment which included 3-4 daily applications for 2 days or through daily applications for periods of 10 and 14 days [10]. In another study conducted comparing the effectiveness of 0.12% chlorhexidine in mouthwash and 0.2% chlorhexidine in gel it was found that the gel had a lower incidence of alveolar osteitis than the mouthwash, 7.5% versus 25% [5].
Varnish
Chlorhexidine varnish is often used after scaling and root planning to treat chronic periodontists as it last longer than mouthwash [2]. Chlorhexidine varnish displayed large variations in the level and length of decreased Streptococci mutans [10]. Depending on the subject the decrease in Streptococci mutans could last for 3 days to 2 weeks [10]. Also the greater the concentration the greater the decease level in Streptococci mutans.In another study varnish was also found to decrease probing depth, reducing pockets by between 0.70 and 1.37 mm [2]
Conclusion
Chlorhexidine varnish and gel were the most effective out of the three types of chlorhexidine treatments studied [5] [10]. Out of the two chlorhexidine varnish was found to be more effective than the gel [10].
Alternatives to Chlorhexidine
Though chlorhexidine is an effective way of treating periodontal diseases it does have negative side effects namely bad odor, a slight staining in teeth, and a change in taste perception [3] [4].Chlorhexidine reduced the taste intensity of NaCl to 35 ± 5% and quinine HCl to 50 ± 8% of pretreatment strength [3]. There was no effect on sucrose; the mean posttreatment intensity was 97 ± 5% of pretreatment but citric acid intensity increased to 139 ± 13% [3].There have studies that found alternatives which work just as well as chlorhexidine without the negative effects.
Triphala mouthwash
Triphala is a combination of three different plant extracts (Amalaki, Bibhitaki, and Haritaki). used in a mouthwash to treat diseases because of its antimicrobial and antioxidant properties [9]. Triphala was found to be as effective as 2% chlorhexidine in treating plaque [9].
Terminalia chebula
The plant extract of Terminalia chebula was compared with chlorhexidine to see if it would be a good alternative to treat dental caries, gingivitis and stomatitis [4]. There was a significant decrease in plaque in both the chlorhexidine group and Terminalia chebula [4]. There was no significant difference between both group and both reduced plaque buildup and gingivitis [4].
Further Reading
Though chlorhexidine has its benefits it might also cause heart disease. In a study conducted in 2013, they used mouthwash which contained chlorhexidine to suppress oral microbes to determine if it affects systemic nitrite levels and blood pressure in healthy individuals, as nitrite is found to be a precursor for systemic generation of vasodilatory nitric oxide, and external administration of nitrate reduces blood pressure in humans [6].
References
1 Adams, D., and M. Addy. "Mouthrinses." Advances in Dental Research 8.2 (1994): 291. Web.
Edited by (Martha Serrano), a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.