THE DIAGNOSIS OF SYPHILIS: Difference between revisions
No edit summary |
|||
| (58 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==Introduction== | ==Introduction== | ||
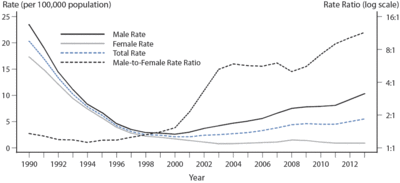
<br>By | [[Image:Syphilis prevalence 2.gif|thumb|400px|right|Women and men ratios of syphilis prevalence. It shows the rise after year 2000. Figure 32. Primary and Secondary Syphilis — Rates of Reported Cases by Sex and Male-to-Female Rate Ratios, United States, 1990–2013.[http://www.cdc.gov/std/stats13/figures/32.htm].]] | ||
<br>By Heather Fantry<br> | |||
Syphilis is a sexually transmitted infection that has been rising in prevalence since 2000 (CDC, 2013). | Syphilis is a sexually transmitted infection that is caused by the spirochete <i>Treponema pallidum</i>. It has been rising in prevalence in the United States since 2000 (Figure 1) (CDC, 2013). This rise is primarily fueled by an increase in spread among men who have sex with men but there also have been a rise of cases in women and even infants. Syphilis is even more of a health risk in developing countries. It is estimated that there are 10.6 million new cases of syphilis worldwide each year (WHO, 2008). Although it is effectively treated with penicillin, the disease continued to spread in all parts of the world because of the difficulties in diagnosing syphilis. | ||
== | ==Clinical Signs and Symptoms== | ||
[[Image:chancre-penile-1.jpg|thumb|300px|right|Syphilis penis. Primary stage of syphilis (chancre) on glans (head) of the penis.[http://www.cdc.gov/std/syphilis/images/chancre-penile.htm].]] | [[Image:chancre-penile-1.jpg|thumb|300px|right|Syphilis penis. Primary stage of syphilis (chancre) on glans (head) of the penis.[http://www.cdc.gov/std/syphilis/images/chancre-penile.htm].]] | ||
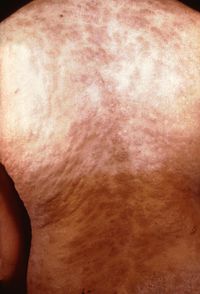
[[Image:Second stage syphilis.jpg |thumb| | [[Image:Second stage syphilis.jpg |thumb|200px|right|Secondary stage of syphilis Rash on back.[http://www.cdc.gov/std/syphilis/images/rash-gbr.htm].]]The clinical presentation of syphilis can make diagnosis difficult. Primary syphilis h occurs 9-90 days after contact with <i>T. pallidum</i> (Tramont, 2005). It is manifested by a skin lesion called a chancre (Figure 2) (CDC, 2013). It starts out as flat area of redness that develops into a bump and then into a swallowing opening in the skin. The chancre occurs at any place on the body in which an individual has had contact with <i>T. pallidum</i>. This is usually on the penis in a man or external genitalia in a woman but can be in the mouth or even on a finger. Because this lesion is painless and may occur in hidden areas of the body like on the anus or inside the labia, the lesion of primary syphilis can be missed. | ||
The | |||
The | The next stage, secondary syphilis, is usually not missed because <i>T. pallidum</i> has spread throughout the body and has a variety of manifestations. Typically, this occurs 3-10 weeks after the initial lesion if a person is not treated with antibiotics (Tramont, 2005). The most common symptom is a non-itchy rash that is not raised and often is on all parts of the body (Figure 3). If the rash is on the palms and soles, this is especially helpful for diagnosis since syphilis is one of the few diseases that cause rash in these parts of the body. Individuals also may develop condyloma latum, flat bumps in the genital area, and mucous patches, flat patches in the mouth, which are characteristic of syphilis. However, rashes associated with syphilis can be atypical and less than half of patients develop condyloma latum and mucous patches. Other manifestations such as fever, headache, weight loss, lymph node swelling, loss of hair, and eye disease are seen in many other diseases and, unless a patient or clinician is suspicious of syphilis, can be attributed to other conditions. | ||
The final stage | The next stage of syphilis, latent syphilis, is even more difficult to diagnose since, although <i>T. pallidum</i> remains in the body, there are no outward manifestations of disease. Only a positive blood test will indicate that an individual has syphilis (CDC, 2013). | ||
The final stage, tertiary syphilis, only occurs in 35% of untreated patients and occurs 10-25 years after primary syphilis so it is often unsuspected. Clinicians are often are unaware of past sexual histories that may suggest the diagnosis. It can cause severe neurological disease which may include meningitis; strokes; dysfunction of cranial nerves resulting in problems with vision, eye movement, and hearing; dementia; difficulty walking; and loss of vibration sense. Tertiary syphilis can also cause destructive lesions of the skeleton, spine, mucosal areas, eyes, and organs caused by gummas, growths of granulomatous tissue, which are distinctive but rarely seen. Finally, it can cause heart disease including aortic aneurysms, aortic valve abnormalities, and narrowing of blood vessels but these are not specific only to syphilis and are also rarely seen. | |||
Besides infecting sexual partners, a woman with syphilis can infect her unborn child. Although rare in the United States, 2 million pregnant women worldwide are infected with <i>T. pallidum</i> each year and, if undiagnosed, and transmit infection to their unborn children (Shahrook et al, 2014). Congenital syphilis can cause deafness, neurological problems, bone deformities, and even death (CDC, 2013). | |||
<br> | <br> | ||
<br> | <br> | ||
== | ==Who Should be Tested for Syphilis?== | ||
Since clinical manifestations of disease do not always confirm the diagnosis, the United States Center for Disease Control and Prevention (CDC) has guidelines regarding who to test for syphilis (CDC, 2014). The first group is the most obvious and includes any individuals with signs and symptoms of syphilis (CDC, 2014). The second group is individuals with no signs or symptoms of syphilis but who are at risk. This includes persons whose have multiple sexual partners, use illegal drugs, use alcohol, have unprotected sex, and are prostitutes. It also includes men who have sex with men, prisoners, HIV-infected patients, and, individuals who have sexual partners who has syphilis. Furthermore, women who are pregnant should also be tested because of the risk of transmission to their unborn child. | |||
The CDC also has recommendations for testing based on where a person lives. The states in the southern United States, Maryland and California have higher rates than states in the central United States (CDC, 2013). Urban areas also have higher rates than urban areas. It is recommended that there should be a lower threshold for and more frequent testing in high prevalence areas. | |||
The CDC guidelines, especially in pregnant women who come for prenatal care, are helpful in increasing the number of cases of syphilis diagnosed but they still present challenges to diagnosis. Many of the individuals who are at high risk for syphilis such as drug users and prostitutes do not have or choose not to have access health care and testing. | |||
<br> | <br> | ||
<br> | <br> | ||
== | ==Direct Diagonsis== | ||
<br> Even when individuals who have signs and symptoms or are at high risk of syphilis are brought in for testing, diagnosis is still challenging. Unlike most bacteria that infect humans, <i>T. pallidum</i> cannot be cultured in the laboratory and only can be cultured in laboratory animals. The most commonly used animals, rabbits, are not readily found in health care facilities or labs where syphilis needs to be diagnosed. | |||
<b>Microscopy</b> | |||
<i>T. pallidum</i>, although it cannot be cultured, can be seen under a microscope. This is the most sensitive and quickest method to diagnose primary syphilis (Tramont 2005). A swab is place in the chancre and <i>T. pallidum</i> attaches to the swab and can be viewed under a microscope. Under a microscopy, <i>T. pallidum</i> looks like a corkscrew rapidly turning around its midpoint (Figure 4) (Slonczewski and Foster, 2014 and Tramont, 1995). However, <i>T. pallidum</i> is so thin (0.1 µm) that it cannot resolve light and thus can only be detected by light scattering which requires darkfield microscopy. A ark field microscope requires skilled technicians and is expensive so most health care facilities, even in developed nations, do not have them (Tramont, 1995). [[Image:darkfeild-1.jpg |thumb|300px|right| Darkfield microscopy of Syphilis.[http://www.cdc.gov/std/syphilis/images/treponema-pallidum.htm].]] | |||
<b>Polymerase Chain Reaction (PCR)</b> | |||
Another direct method fraught with the same problems of lack of ability and expense is PCR. PCR can be used to amplify sections of <i>T. pallidum</i> DNA which was fully sequenced in 1998 (Fraser et al, 1998). It can be used to diagnose primary syphilis by taking a swab from the chancre. This method has been showed to be 82% sensitive and 95% specific (Grange et al, 2012). It can also be used to diagnose secondary syphilis using a blood sample and to diagnose congenital syphilis using frozen or formalin fixed placental tissue (Genest et al, 1996 and Heymans et al, 2010). <i>T. pallidum</i> PCR, however, is not readily available in the United States and specimens have to be shipped to the CDC (CDC, 2015). Submission requires special approval by the local health department and the turnaround time is two weeks. | |||
<br> | |||
<br> | <br> | ||
==Antibody Tests== | |||
Since dark field microscopy and PCR are not readily available, clinicians need to rely on indirect methods of diagnosis. These indirect methods may produce false negatives and false positives (Lab Tests Online, 2013). Despite these limitations, antibody tests are the primary methods used to diagnose syphilis today. | |||
<b>Nontreponemal Antibody Tests</b> | |||
<br>Some of the oldest and most widely used antibodies tests are the nontreponemal antibody tests. However, instead of measuring antibodies directed against the pathogen, as most antibody tests do, these tests measure antibodies against a product that is produced when <i>T. pallidum</i> interacts with human tissue (CDC, 2013). This product is cardiolipin-lecithin–cholesterol antigen. The antibodies measured are both IgG and IgM antibodies. The most commonly used nontreponemal antibody tests are the Rapid Plasma Reagin (RPR), Venereal Disease Research Laboratory (VDRL), toluidine red unheated test (TRUST), and Unheated Serum Reagin (USR) (CDC, 2013). Both the RPR and VDRL are quantitative results (CDC, 2013). High titers such as 1:128 mean that there is more antibody present than low titers such as 1:2. The titer is used to determine whether or not an individual has been effectively treated. | |||
One problem with using antibody tests to diagnose an infection is that it takes time to develop antibodies and antibodies can decrease with time. In primary syphilis, 14% of RPR and 22% of VDRL tests are negative in persons who have syphilis (CDC, 2013). In tertiary syphilis, many years after the initial infection, 27% of RPR and 29% of VDRL tests are negative in persons with syphilis. This is not a problem in secondary syphilis and less of a problem in latent syphilis since antibodies have had time to develop and have not waned with time. | |||
Another problem is that antibody tests can be nonspecific. Many antibody tests are positive when there is a closely related pathogen in an individual. This can happen in the case of syphilis if an individual has infection such as Yaws which is caused by another species of Treponema (CDC, 2013). However, there are other reasons for false positives for the nontreponemal antibody tests. Since they are directed against-cardiolipin-lecithin–cholesterol antigen, any processes in the body that produces this antigen can result in a positive test. This includes other bacterial infections, viral infections including HIV, injection drug use, vaccination, and autoimmune diseases. | |||
<b>Treponemal Immunofluorescent Antibody Tests</b> | |||
<br>Because of the lack of specificity of the nontreponemal antibody tests, all positive nontreponemal tests need to be confirmed with a more specific test. The more specific tests measure antibodies directed at the pathogen, <i>T. pallidum</i>. They do not eliminate the problem of false negative results in early infection but they do increase specificity since, although species of Treponema may give false positive results, other processes like autoimmune diseases do l not. However, these tests are not quantitative so the effectiveness of treatment. | |||
Until recently, there were only three antibody tests that specifically measured antibodies to <i>T. pallidum</i> (Tramont, 2005). These were the fluorescent treponemal antibody-absorbed (FTA-abs), <i>T. pallidum</i> Haemagglutination Assay (TPHA) and Microhemagglutination Assay for Antibodies to <i>T. pallidum</i> (MHATP). The most commonly used test is the FTS-abs. It uses <i>T. pallidum</i> taken from rabbit testes and is a standard immunofluorescent antibody test in which an antibody is labeled with a compound that fluoresces. Since nonpathogenic treponemes are in the mouth and genital tract of humans, the antibodies against these treponemes need to be removed by a “sorbent” which is a nonpathogenic antigen. The patient’s serum is then placed on slide with a <i>T. pallidum</i> antigen and fluorescein-labeled antihuman gamma globulin is added to the slide. If there are antibodies to <i>T. pallidum</i>, the slide will fluoresce when viewed in fluorescence microscope. | |||
<b>Enzyme-linked Immunosorbent Assay (ELISA)</b> | |||
<br>Recently, another type of antibody test that detects antibodies against <i>T. pallidum</i>, an ELISA test, has come into widespread use (CDC, 2008, CDC 2010). It performs better in primary syphilis and when done in large quantities, it is less expensive than nontreponemal antibody tests. It is performed by coating the wells of a plastic laboratory dish with <i>T. pallidum</i> (Slonczewski and Foster, 2014). If antibodies to <i>T. pallidum</i> are present, then they will bind with the <i>T. pallidum</i>. Unbound antibodies in the serum are washed away and another antibody linked to an enzyme that binds to the <i>T. pallidum</i> antibodies is added to the dish. When a substrate for the enzyme is placed in the dish, the enzyme produces light or a colored product only in the wells that have antibody to <i>T. pallidum</i>. There will be no light in the wells without <i>T. pallidum</i> antibodies. | |||
Because ELISA tests to diagnose syphilis are less expensive than nontreponemal antibody tests when performed on large numbers of samples, some sexually transmitted disease clinics and blood banks screen with an ELISA test (CDC, 2008, CDC, 2010). Unlike nontreponemal antibody tests, there is give no titer so they cannot distinguish between appropriately treated past infection and infection that requires treatment (CDC, 2010). It is recommended that a nontreponemal test be used for confirmation and to make treatment decisions. | |||
<b>Reverse enzyme-linked immunospot assay (RELISPOT)</b> | |||
<br>In the late 1990, enzyme linked immunospot assays were developed in an attempt to differentiate active syphilis from treated syphilis (Tabidze, 1999). In these assays, blood mononuclear cells are collected and tested for <i>T. pallidum</i>-specific circulating antibody-secreting cells by an enzyme-linked immunospot assay (ELISPOT). This type of assay enables visualization of products secreted by human immune cells (Wikipedia, 2014). Each spot on the assay represents a single reactive cell. Some early work showed that they were positive in 100% of patients with primary syphilis, 87% of patients with secondary syphilis, and 46% of patients with latent syphilis. However, there is no mention of this test in later papers or in current recommendations for diagnosing syphilis (CDC, 2014, CDC, 2013, CDC, 2010). | |||
This tests is based on a localized enzyme-substrate reaction using petri dishes coated with antibodies (Czerkinsky et al, 1984). Similar to the ELISPOT, the RELISPOT can be used to give a quantitation of secreted antigen. Its major use in syphilis is to distinguish active infection in the newborn from passive transfer of antibodies from the mother to the infant (Stoll et al, 1993). Stoll et al found that sensitivities of the RELISPOT were not as high as the IgM ELISA or FTA-abs but its specificity was >96%. | |||
<b>Point-of-Care Testing</b> | |||
<br>Another type of antibody testing has been developed in the last 20 years. These tests can be done at the site where a patient receives medical care. They do not require a specialized laboratory and reading the test only requires minimal training. Furthermore, these tests are inexpensive. Most importantly, results are available in 15 minutes or less so that patients can be notified immediately of their results. Since many health care facilities in developing countries do not have access to specialized laboratories and many patients in both developing and developed countries do not return for treatment, these tests have the potential to increase testing as well as increase appropriate treatment. | |||
Multiple observational studies have provided data that suggest that point-of-care testing is superior to traditional methods. For example, from August 2008 to May 2009, Mishra and colleagues undertook a prospective study to determine whether point of care testing enhanced the ability to diagnose syphilis. They followed women sex workers in Bangalore, India and compared point-of-care testing with traditional testing. All women had no prior diagnosis of syphilis and attended the same sexually transmitted disease (STD). 1617 women received point-of-care testing instead of the standard RPR screening. The women who received point-of-care testing were treated immediately. The number of women that received appropriate treatment rose from 44.8% to 68.3%. | |||
Randomized studies on the effectiveness of point-of-care testing compared to traditional testing are limited but the Cochrane Database recently reported the results of two studies comparing the two testing strategies for diagnosing, treating, and preventing infection in unborn children (Shahrook, 2014). The outcomes of the two studies were assessed differently so a meta-analysis could not be performed. The two trials included 8493 pregnant women. One of the studies, conducted in primary care clinics in rural South Africa, randomized clinics to perform either point-of-care or traditional testing. This study found no difference in treatment of perinatal mortality in the 793 women who tested positive for syphilis. It is possible that randomization by clinic instead of by individual or that perinatal mortality was affected by other factors rather than syphilis may have resulted in these negative findings. In addition, there were no data reported on the 6,825 women in the study who did not test positive for syphilis. Furthermore, secondary outcomes which might have caused differences in perinatal mortality were not assessed. | |||
By contrast, another study of 7700 pregnant women from 14 antenatal clinics in Ulaanbaata, Mongolia found that the percentage of women tested and treated for syphilis was higher in women who had point-of-care testing. Furthermore, a higher proportion of partners were treated and there were a smaller proportion of children who contracted syphilis. Although this study also had limitations such as unclear randomization methods and no reporting of other health conditions like HIV that could influence outcomes, it suggests that point-of-care testing is beneficial. | |||
There are many types of point-of-care tests. A modified RPR test can be done at the point-of-care (Peeling et al, 2006). There is also a test called the line immunoassay (LIA) that can be done at the point-of-care. The LIA uses <i>T. pallidum</i> recombinant and synthetic polypeptide antigens to determine if a patient’s blood has treponemal antibodies (Hagedon et al, 2002). The sensitivity and specificity of the LIA are 100 % and 99 %, respectively (Hagedon et al, 2002). | |||
Another group of point-of-care tests are the immunochromatographic tests which are immunochromatographic membrane tests (ICT) or immunochromatographic strips (ICS). Serum is placed on a cellulose strip bound with anti-human immunoglobulins and/or purified <i>T. pallidum</i> antigens (Slonczewski and Foster, 2014). If there are antibodies in the patient’s serum, there is a color change which is visible to the naked eye. Sensitivity and specificity are between 85% and 98% when compared to other antibody tests (Herring A, 2006, Slonczewski and Foster, 2014). | |||
Until recently, none of these tests were approved by use in the United States so they were used primarily in developing countries. However, in 2014, the U.S. Food and Drug Administration (FDA) approved an immunochromatographic test called Syphilis Health Check (U.S. Food and Drug Administration, 2014). The manufactures of this test claim 98% agreement with other treponemal tests (Diagnostics direct, 2011). | |||
Finally, there is another point-of- care test that is under investigation is test which combines treponemal and nontreponemal antibody tests. Hence there is no need for a confirmatory test. The test is called the DPP Syphilis Screen and Confirmation Assay. A recent study showed that the sensitivity was 89.8% and specificity was 99.3% when compared with immunoassay and RPRs (Causer, 2015). | |||
==Interpreting Antibody Tests== | |||
<br>As mention in the prior section, if a nontreponemal antibody test is positive, then it should be confirmed with a treponemal antibody test and if a treponemal antibody test is positive, then it should be confirmed with a nontreponemal antibody test (CDC, 2010). The latter is called reverse sequence screening. However, even when this is done, it is still not easy to differentiate a new case of syphilis that needs treatment from a prior case of syphilis that does not. This is because, unlike many infections, a person with syphilis can be infected more than once and, in fact, 20% of persons with syphilis have had at least one prior episode. Treponemal tests for most of these individuals will remain reactive for life even if they have received appropriate treatment (CDC, 2010). In contrast, the nontreponemal tests (RPR and VDRL) in most people revert to nonreactive. This makes it essential to get a nontreponemal test to determine whether or not to treat. | |||
It is even more difficult to diagnose active disease in the 1-4% of patients who remain serofast (CDC 2010. This means that the RPR pr VDRL test remains reactive. The only way to know if it is an infection that needs treatment is to compare titers. If the titer is four fold lower than pervious (i.e. 1:128 down to 1:32), this means that the person has been appropriately treated and no further therapy is needed (CDC, 2010). If the titer has risen, did not change or only decreased two fold (1:128 to 1:64), then the person has not had appropriate treatment or has been reinvented. Repeat testing should be done with the same test (either an RPR or VDRL) and ideally done by the same lab. RPR titers are often higher than VDRL titers. | |||
Although there have been reports of unusually high or low or changing titers in HIV-infected persons, tests should be performed and interpreted in the same manner for HIV-infected individuals as for non-HIV-infected individuals (CDC 2010). | |||
==Testing for Neurosyphilis== | |||
The diagnosis of neurosyphilis is less direct and even more challenging than the diagnosis of other forms of syphilis. In 30% of cases of primary and secondary syphilis, <i>T. pallidum</i> does invade the cerebrospinal fluid but there is no obvious clinical signs or symptoms and no long term sequel. Therefore, it is not recommended to test for neurosyphilis routinely in individuals with primary and secondary syphilis. | |||
However, some individuals, especially AIDS patients and other immunocompromised individuals, develop neurosyphilis. It is important to make this diagnosis because it can cause significant morbidity and mortality and it needs to be treated differently than other forms of syphilis. The antibiotics need to be given in high enough doses so that they penetrate the central nervous system. | |||
Besides testing for nontreponemal and treponemal antibodies, persons with signs and symptoms of neurosyphilis, those who do not respond to treatment, and those with tertiary syphilis manifested by heart disease or gummas need to have lumbar puncture. A lumbar puncture is when a needle is inserted between the bones in the lumbar spine to remove cerebrospinal fluid (CSF), the fluid surrounding the brain (Mayo Clinc Staff, 2015). Certain tests need to be performed on the CSF. These include a VDRL, one of the nontreponemal antibody tests; a cell count to determine how many and what type of white blood cells; and protein level. The VDRL is highly specific but is not very sensitive so many individuals that have neurosyphilis do not have a positive CSF-VDRL. It the CSF-VDRL is negative, a white blood cell count >5 cells/mm3 or a protein level ≥ 46 mg/dl are suggestive of neurosyphilis (CDC, 2013). However, these tests are not specific, especially in HIV patients, who often have >5 white blood cell count /mm3 in the CDF (CDC, 2010). | |||
==Testing for Congenital Syphilis== | |||
It is recommended that all pregnant women should be tested for syphilis at the first prenatal visit and then repeated again during the third trimester (CDC, 2012). High risk women or those living in area with a high prevalence of syphilis should be tested again in third trimester and a delivery. Testing of the mother is done with nontreponemal and treponemal antibody tests as in other non-pregnant adults. | |||
If syphilis is diagnosed during the second half of pregnancy in the mother, than the infant should immediately have an ultrasound to look for signs of syphilis such as live enlargement, fluid in body compartments, or a thickened placenta (CDC, 2010). If an infant is born to a mother with a reactive nontreponemal and treponemal, then the infant needs to be evaluated for congenital syphilis (CDC, 2014). The infant’s serum should be tested with a nontreponemal test but the infant’s blood may not be reactive especially if the mother has low titers or the mother was recently infected (CDC, 2010). The infant should also be examined for evidence of syphilis. Any skin lesions that are suspicious should be swabbed. Swabs from these lesions and other body fluids, and tissue from birth such as the placenta or umbilical cord should be examined under a darkfield microscope for <i>T. pallidum</i>. These specimens can also be examined for IgM specific antibodies using ELISA, Relispot, FTA-abs, or immunoblotting/Western blot. Specimens should be sent to the CDC for PCR (CDC, 2010). A lumbar puncture can also help establish the diagnosis. The same tests (VDRL, cell count and protein) are done as in adults who may have neurosyphilis. X-rays of the long bones and an ultrasound to look for abnormal fluid are also helpful. | |||
==Conclusion== | |||
Because of missed primary lesions, misdiagnosed secondary and tertiary signs and symptoms, lack of signs and symptoms during latent syphilis, and our inability to grow <i>T. pallidum</i> in the laboratory, syphilis can be challenging to diagnose. Direct testing, even with PCR has not provided much improvement in diagnosis. However, there are new point-of- care tests that will increase the availability and speed of antibody testing so that treatment can be given to more individuals with syphilis in the hope of slowing down or even halting this epidemic. | |||
==References== | ==References== | ||
1. Causer LM, et al. An Evaluation of a Novel Dual Treponemal/Nontreponemal Point-of-Care Test for Syphilis as a Tool to Distinguish Active From Past Treated Infection. Clin Infect Dis. 2015 Mar 25. pii: civ243. | |||
<br>2. CDC. Sexually Transmitted Treatment Guidelines 2010. MMWR.2010;59:1-110. | |||
<br>3. CDC. Submitting Specimens to the CDC. http://www.cdc.gov/laboratory/specimen-submission/detail.html?CDCTestCode=CDC-10176. Last updated Arpil 13, 2015. | |||
<br>4. CDC. Syphilis CDC Fact Sheet http://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm. Last updated July 8, 2014. | |||
<br>5. CDC. Syphilis testing algorithms using treponemal tests for initial screening--four laboratories, New York City, 2005-2006.MMWR Morb Mortal Wkly Rep. 2008;57:872 | |||
<br>6. CDC. Syphilis Curriculum. http://www2a.cdc.gov/stdtraining/ready-to-use/syphilis.htm. Lasted updated August 2013. | |||
<br>7. Czerkinsky CC, et al.Reverse enzyme-linked immunospot assay (RELISPOT) for the detection of cells secreting immunoreactive substances. J Immunol Methods. 1984;72:489-96. | |||
<br>8. Diagnostics Direct. 2011. Keep diagnostic tests & revenue in your office. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426843.htm | |||
<br>9. Fraser CM, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375-88. | |||
<br>10. Genest, et al. Hum Pathol. 1996;27:366-72. | |||
<br>11. Grange PA, et al. Evaluation of a PCR test for detection of Treponema pallidum in swabs and blood. J Clin Microbiol. 2012;50:546-52. | |||
<br>12. Hagedorn HJ, et al. Evaluation of INNO-LIA syphilis assay as a confirmatory test for syphilis. J Clin Microbiol 2002;40:973-8. | |||
<br>13. Herring A, et al. WHO/TDR Sexually Transmitted Diseases Diagnostics Initiative. Evaluation of rapid diagnostic tests: syphilis. Nat Rev Microbiol. 2006; 4:S33-40. | |||
<br>14. Heymans R, et al. Clinical value of Treponema pallidum real-time PCR for diagnosis of syphilis. J Clin Microbiol. 2010;48:497-502. | |||
<br>15. Lab Tests Online. 2013. Syphilis Tests. http://labtestsonline.org/understanding/analytes/syphilis/tab/test/ | |||
<br>16. Mayo Clinic Staff. 2015. Lumbar Puncture (spinal tap). http://www.mayoclinic.org/tests-procedures/lumbar-puncture/basics/definition/prc-20012679 | |||
<br>17. Peeling RW, Hook EW 3rd. The pathogenesis of syphilis: the great mimicker, revisited. J Pathol 2006;208:224-32, 2014). | |||
<br>18. Shahrook S, Mori R, Ochirbat T, Gomi H. Strategies of testing for syphilis during pregnancy. Cochrane Database Syst Rev. 2014;10:CD010385. doi: 10.1002/14651858.CD010385.pub2. | |||
<br>19. Slonczewski, J L and Foster JW. Microbiology An Evolving Science. 3rd ed. New York: W. W. Norton & Company 2014. | |||
<br>20. Stoll BJ, et al. Clinical and serologic evaluation of neonates for congenital syphilis: a continuing diagnostic dilemma. . J Infect Dis. 1993;167:1093-9. | |||
<br>21. Tabidze IL, et al. Enzyme-linked immunospot assay for the diagnosis of active Treponema pallidum infection during the various stages of syphilis. Sex Transm Dis. 1999;26:426-30. | |||
<br>22. Tramont EC. Syphilis in adults: from Christopher Columbus to Sir Alexander Fleming to AIDS. Clin Infect Dis. 1995;21:1361-71. | |||
<br>23. Tramont EC. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. 6th ed. New York: Churchill Livingston 2005:2768-85. | |||
<br>24. US Food and Drug Administration. 2014. FDA news Release. FDA grants CLIA waiver expanding the availability of rapid screening test for syphilis. http://www.fda.gov/NewsEvents/Newsroom/Pre | |||
<br>25. WHO. 2008. Global incidence an prevalence of selected curable sexually transmitted infections. http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf | |||
<br>26. Wikipedia. ELISPOT. http://en.wikipedia.org/wiki/ELISPOT. Updated Dec. 4, 2014 | |||
<br>27. Yin YP, et al. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: a multisite evaluation study in China. Clin Infect Dis. 2013;56:659-65. | |||
<br><br>Authored for [http://biology.kenyon.edu/courses/biol238/biol238syl15.html BIOL 238 Microbiology], taught by [mailto:slonczewski@kenyon.edu Joan Slonczewski], 2015, [http://www.kenyon.edu/index.xml Kenyon College]. | <br><br>Authored for [http://biology.kenyon.edu/courses/biol238/biol238syl15.html BIOL 238 Microbiology], taught by [mailto:slonczewski@kenyon.edu Joan Slonczewski], 2015, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
Latest revision as of 15:22, 1 October 2015
Introduction
By Heather Fantry
Syphilis is a sexually transmitted infection that is caused by the spirochete Treponema pallidum. It has been rising in prevalence in the United States since 2000 (Figure 1) (CDC, 2013). This rise is primarily fueled by an increase in spread among men who have sex with men but there also have been a rise of cases in women and even infants. Syphilis is even more of a health risk in developing countries. It is estimated that there are 10.6 million new cases of syphilis worldwide each year (WHO, 2008). Although it is effectively treated with penicillin, the disease continued to spread in all parts of the world because of the difficulties in diagnosing syphilis.
Clinical Signs and Symptoms
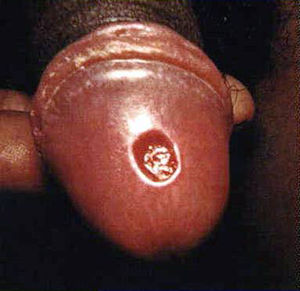
The clinical presentation of syphilis can make diagnosis difficult. Primary syphilis h occurs 9-90 days after contact with T. pallidum (Tramont, 2005). It is manifested by a skin lesion called a chancre (Figure 2) (CDC, 2013). It starts out as flat area of redness that develops into a bump and then into a swallowing opening in the skin. The chancre occurs at any place on the body in which an individual has had contact with T. pallidum. This is usually on the penis in a man or external genitalia in a woman but can be in the mouth or even on a finger. Because this lesion is painless and may occur in hidden areas of the body like on the anus or inside the labia, the lesion of primary syphilis can be missed.
The next stage, secondary syphilis, is usually not missed because T. pallidum has spread throughout the body and has a variety of manifestations. Typically, this occurs 3-10 weeks after the initial lesion if a person is not treated with antibiotics (Tramont, 2005). The most common symptom is a non-itchy rash that is not raised and often is on all parts of the body (Figure 3). If the rash is on the palms and soles, this is especially helpful for diagnosis since syphilis is one of the few diseases that cause rash in these parts of the body. Individuals also may develop condyloma latum, flat bumps in the genital area, and mucous patches, flat patches in the mouth, which are characteristic of syphilis. However, rashes associated with syphilis can be atypical and less than half of patients develop condyloma latum and mucous patches. Other manifestations such as fever, headache, weight loss, lymph node swelling, loss of hair, and eye disease are seen in many other diseases and, unless a patient or clinician is suspicious of syphilis, can be attributed to other conditions.
The next stage of syphilis, latent syphilis, is even more difficult to diagnose since, although T. pallidum remains in the body, there are no outward manifestations of disease. Only a positive blood test will indicate that an individual has syphilis (CDC, 2013).
The final stage, tertiary syphilis, only occurs in 35% of untreated patients and occurs 10-25 years after primary syphilis so it is often unsuspected. Clinicians are often are unaware of past sexual histories that may suggest the diagnosis. It can cause severe neurological disease which may include meningitis; strokes; dysfunction of cranial nerves resulting in problems with vision, eye movement, and hearing; dementia; difficulty walking; and loss of vibration sense. Tertiary syphilis can also cause destructive lesions of the skeleton, spine, mucosal areas, eyes, and organs caused by gummas, growths of granulomatous tissue, which are distinctive but rarely seen. Finally, it can cause heart disease including aortic aneurysms, aortic valve abnormalities, and narrowing of blood vessels but these are not specific only to syphilis and are also rarely seen.
Besides infecting sexual partners, a woman with syphilis can infect her unborn child. Although rare in the United States, 2 million pregnant women worldwide are infected with T. pallidum each year and, if undiagnosed, and transmit infection to their unborn children (Shahrook et al, 2014). Congenital syphilis can cause deafness, neurological problems, bone deformities, and even death (CDC, 2013).
Who Should be Tested for Syphilis?
Since clinical manifestations of disease do not always confirm the diagnosis, the United States Center for Disease Control and Prevention (CDC) has guidelines regarding who to test for syphilis (CDC, 2014). The first group is the most obvious and includes any individuals with signs and symptoms of syphilis (CDC, 2014). The second group is individuals with no signs or symptoms of syphilis but who are at risk. This includes persons whose have multiple sexual partners, use illegal drugs, use alcohol, have unprotected sex, and are prostitutes. It also includes men who have sex with men, prisoners, HIV-infected patients, and, individuals who have sexual partners who has syphilis. Furthermore, women who are pregnant should also be tested because of the risk of transmission to their unborn child.
The CDC also has recommendations for testing based on where a person lives. The states in the southern United States, Maryland and California have higher rates than states in the central United States (CDC, 2013). Urban areas also have higher rates than urban areas. It is recommended that there should be a lower threshold for and more frequent testing in high prevalence areas.
The CDC guidelines, especially in pregnant women who come for prenatal care, are helpful in increasing the number of cases of syphilis diagnosed but they still present challenges to diagnosis. Many of the individuals who are at high risk for syphilis such as drug users and prostitutes do not have or choose not to have access health care and testing.
Direct Diagonsis
Even when individuals who have signs and symptoms or are at high risk of syphilis are brought in for testing, diagnosis is still challenging. Unlike most bacteria that infect humans, T. pallidum cannot be cultured in the laboratory and only can be cultured in laboratory animals. The most commonly used animals, rabbits, are not readily found in health care facilities or labs where syphilis needs to be diagnosed.
Microscopy
T. pallidum, although it cannot be cultured, can be seen under a microscope. This is the most sensitive and quickest method to diagnose primary syphilis (Tramont 2005). A swab is place in the chancre and T. pallidum attaches to the swab and can be viewed under a microscope. Under a microscopy, T. pallidum looks like a corkscrew rapidly turning around its midpoint (Figure 4) (Slonczewski and Foster, 2014 and Tramont, 1995). However, T. pallidum is so thin (0.1 µm) that it cannot resolve light and thus can only be detected by light scattering which requires darkfield microscopy. A ark field microscope requires skilled technicians and is expensive so most health care facilities, even in developed nations, do not have them (Tramont, 1995).

Polymerase Chain Reaction (PCR)
Another direct method fraught with the same problems of lack of ability and expense is PCR. PCR can be used to amplify sections of T. pallidum DNA which was fully sequenced in 1998 (Fraser et al, 1998). It can be used to diagnose primary syphilis by taking a swab from the chancre. This method has been showed to be 82% sensitive and 95% specific (Grange et al, 2012). It can also be used to diagnose secondary syphilis using a blood sample and to diagnose congenital syphilis using frozen or formalin fixed placental tissue (Genest et al, 1996 and Heymans et al, 2010). T. pallidum PCR, however, is not readily available in the United States and specimens have to be shipped to the CDC (CDC, 2015). Submission requires special approval by the local health department and the turnaround time is two weeks.
Antibody Tests
Since dark field microscopy and PCR are not readily available, clinicians need to rely on indirect methods of diagnosis. These indirect methods may produce false negatives and false positives (Lab Tests Online, 2013). Despite these limitations, antibody tests are the primary methods used to diagnose syphilis today.
Nontreponemal Antibody Tests
Some of the oldest and most widely used antibodies tests are the nontreponemal antibody tests. However, instead of measuring antibodies directed against the pathogen, as most antibody tests do, these tests measure antibodies against a product that is produced when T. pallidum interacts with human tissue (CDC, 2013). This product is cardiolipin-lecithin–cholesterol antigen. The antibodies measured are both IgG and IgM antibodies. The most commonly used nontreponemal antibody tests are the Rapid Plasma Reagin (RPR), Venereal Disease Research Laboratory (VDRL), toluidine red unheated test (TRUST), and Unheated Serum Reagin (USR) (CDC, 2013). Both the RPR and VDRL are quantitative results (CDC, 2013). High titers such as 1:128 mean that there is more antibody present than low titers such as 1:2. The titer is used to determine whether or not an individual has been effectively treated.
One problem with using antibody tests to diagnose an infection is that it takes time to develop antibodies and antibodies can decrease with time. In primary syphilis, 14% of RPR and 22% of VDRL tests are negative in persons who have syphilis (CDC, 2013). In tertiary syphilis, many years after the initial infection, 27% of RPR and 29% of VDRL tests are negative in persons with syphilis. This is not a problem in secondary syphilis and less of a problem in latent syphilis since antibodies have had time to develop and have not waned with time.
Another problem is that antibody tests can be nonspecific. Many antibody tests are positive when there is a closely related pathogen in an individual. This can happen in the case of syphilis if an individual has infection such as Yaws which is caused by another species of Treponema (CDC, 2013). However, there are other reasons for false positives for the nontreponemal antibody tests. Since they are directed against-cardiolipin-lecithin–cholesterol antigen, any processes in the body that produces this antigen can result in a positive test. This includes other bacterial infections, viral infections including HIV, injection drug use, vaccination, and autoimmune diseases.
Treponemal Immunofluorescent Antibody Tests
Because of the lack of specificity of the nontreponemal antibody tests, all positive nontreponemal tests need to be confirmed with a more specific test. The more specific tests measure antibodies directed at the pathogen, T. pallidum. They do not eliminate the problem of false negative results in early infection but they do increase specificity since, although species of Treponema may give false positive results, other processes like autoimmune diseases do l not. However, these tests are not quantitative so the effectiveness of treatment.
Until recently, there were only three antibody tests that specifically measured antibodies to T. pallidum (Tramont, 2005). These were the fluorescent treponemal antibody-absorbed (FTA-abs), T. pallidum Haemagglutination Assay (TPHA) and Microhemagglutination Assay for Antibodies to T. pallidum (MHATP). The most commonly used test is the FTS-abs. It uses T. pallidum taken from rabbit testes and is a standard immunofluorescent antibody test in which an antibody is labeled with a compound that fluoresces. Since nonpathogenic treponemes are in the mouth and genital tract of humans, the antibodies against these treponemes need to be removed by a “sorbent” which is a nonpathogenic antigen. The patient’s serum is then placed on slide with a T. pallidum antigen and fluorescein-labeled antihuman gamma globulin is added to the slide. If there are antibodies to T. pallidum, the slide will fluoresce when viewed in fluorescence microscope.
Enzyme-linked Immunosorbent Assay (ELISA)
Recently, another type of antibody test that detects antibodies against T. pallidum, an ELISA test, has come into widespread use (CDC, 2008, CDC 2010). It performs better in primary syphilis and when done in large quantities, it is less expensive than nontreponemal antibody tests. It is performed by coating the wells of a plastic laboratory dish with T. pallidum (Slonczewski and Foster, 2014). If antibodies to T. pallidum are present, then they will bind with the T. pallidum. Unbound antibodies in the serum are washed away and another antibody linked to an enzyme that binds to the T. pallidum antibodies is added to the dish. When a substrate for the enzyme is placed in the dish, the enzyme produces light or a colored product only in the wells that have antibody to T. pallidum. There will be no light in the wells without T. pallidum antibodies.
Because ELISA tests to diagnose syphilis are less expensive than nontreponemal antibody tests when performed on large numbers of samples, some sexually transmitted disease clinics and blood banks screen with an ELISA test (CDC, 2008, CDC, 2010). Unlike nontreponemal antibody tests, there is give no titer so they cannot distinguish between appropriately treated past infection and infection that requires treatment (CDC, 2010). It is recommended that a nontreponemal test be used for confirmation and to make treatment decisions.
Reverse enzyme-linked immunospot assay (RELISPOT)
In the late 1990, enzyme linked immunospot assays were developed in an attempt to differentiate active syphilis from treated syphilis (Tabidze, 1999). In these assays, blood mononuclear cells are collected and tested for T. pallidum-specific circulating antibody-secreting cells by an enzyme-linked immunospot assay (ELISPOT). This type of assay enables visualization of products secreted by human immune cells (Wikipedia, 2014). Each spot on the assay represents a single reactive cell. Some early work showed that they were positive in 100% of patients with primary syphilis, 87% of patients with secondary syphilis, and 46% of patients with latent syphilis. However, there is no mention of this test in later papers or in current recommendations for diagnosing syphilis (CDC, 2014, CDC, 2013, CDC, 2010).
This tests is based on a localized enzyme-substrate reaction using petri dishes coated with antibodies (Czerkinsky et al, 1984). Similar to the ELISPOT, the RELISPOT can be used to give a quantitation of secreted antigen. Its major use in syphilis is to distinguish active infection in the newborn from passive transfer of antibodies from the mother to the infant (Stoll et al, 1993). Stoll et al found that sensitivities of the RELISPOT were not as high as the IgM ELISA or FTA-abs but its specificity was >96%.
Point-of-Care Testing
Another type of antibody testing has been developed in the last 20 years. These tests can be done at the site where a patient receives medical care. They do not require a specialized laboratory and reading the test only requires minimal training. Furthermore, these tests are inexpensive. Most importantly, results are available in 15 minutes or less so that patients can be notified immediately of their results. Since many health care facilities in developing countries do not have access to specialized laboratories and many patients in both developing and developed countries do not return for treatment, these tests have the potential to increase testing as well as increase appropriate treatment.
Multiple observational studies have provided data that suggest that point-of-care testing is superior to traditional methods. For example, from August 2008 to May 2009, Mishra and colleagues undertook a prospective study to determine whether point of care testing enhanced the ability to diagnose syphilis. They followed women sex workers in Bangalore, India and compared point-of-care testing with traditional testing. All women had no prior diagnosis of syphilis and attended the same sexually transmitted disease (STD). 1617 women received point-of-care testing instead of the standard RPR screening. The women who received point-of-care testing were treated immediately. The number of women that received appropriate treatment rose from 44.8% to 68.3%.
Randomized studies on the effectiveness of point-of-care testing compared to traditional testing are limited but the Cochrane Database recently reported the results of two studies comparing the two testing strategies for diagnosing, treating, and preventing infection in unborn children (Shahrook, 2014). The outcomes of the two studies were assessed differently so a meta-analysis could not be performed. The two trials included 8493 pregnant women. One of the studies, conducted in primary care clinics in rural South Africa, randomized clinics to perform either point-of-care or traditional testing. This study found no difference in treatment of perinatal mortality in the 793 women who tested positive for syphilis. It is possible that randomization by clinic instead of by individual or that perinatal mortality was affected by other factors rather than syphilis may have resulted in these negative findings. In addition, there were no data reported on the 6,825 women in the study who did not test positive for syphilis. Furthermore, secondary outcomes which might have caused differences in perinatal mortality were not assessed.
By contrast, another study of 7700 pregnant women from 14 antenatal clinics in Ulaanbaata, Mongolia found that the percentage of women tested and treated for syphilis was higher in women who had point-of-care testing. Furthermore, a higher proportion of partners were treated and there were a smaller proportion of children who contracted syphilis. Although this study also had limitations such as unclear randomization methods and no reporting of other health conditions like HIV that could influence outcomes, it suggests that point-of-care testing is beneficial.
There are many types of point-of-care tests. A modified RPR test can be done at the point-of-care (Peeling et al, 2006). There is also a test called the line immunoassay (LIA) that can be done at the point-of-care. The LIA uses T. pallidum recombinant and synthetic polypeptide antigens to determine if a patient’s blood has treponemal antibodies (Hagedon et al, 2002). The sensitivity and specificity of the LIA are 100 % and 99 %, respectively (Hagedon et al, 2002).
Another group of point-of-care tests are the immunochromatographic tests which are immunochromatographic membrane tests (ICT) or immunochromatographic strips (ICS). Serum is placed on a cellulose strip bound with anti-human immunoglobulins and/or purified T. pallidum antigens (Slonczewski and Foster, 2014). If there are antibodies in the patient’s serum, there is a color change which is visible to the naked eye. Sensitivity and specificity are between 85% and 98% when compared to other antibody tests (Herring A, 2006, Slonczewski and Foster, 2014).
Until recently, none of these tests were approved by use in the United States so they were used primarily in developing countries. However, in 2014, the U.S. Food and Drug Administration (FDA) approved an immunochromatographic test called Syphilis Health Check (U.S. Food and Drug Administration, 2014). The manufactures of this test claim 98% agreement with other treponemal tests (Diagnostics direct, 2011).
Finally, there is another point-of- care test that is under investigation is test which combines treponemal and nontreponemal antibody tests. Hence there is no need for a confirmatory test. The test is called the DPP Syphilis Screen and Confirmation Assay. A recent study showed that the sensitivity was 89.8% and specificity was 99.3% when compared with immunoassay and RPRs (Causer, 2015).
Interpreting Antibody Tests
As mention in the prior section, if a nontreponemal antibody test is positive, then it should be confirmed with a treponemal antibody test and if a treponemal antibody test is positive, then it should be confirmed with a nontreponemal antibody test (CDC, 2010). The latter is called reverse sequence screening. However, even when this is done, it is still not easy to differentiate a new case of syphilis that needs treatment from a prior case of syphilis that does not. This is because, unlike many infections, a person with syphilis can be infected more than once and, in fact, 20% of persons with syphilis have had at least one prior episode. Treponemal tests for most of these individuals will remain reactive for life even if they have received appropriate treatment (CDC, 2010). In contrast, the nontreponemal tests (RPR and VDRL) in most people revert to nonreactive. This makes it essential to get a nontreponemal test to determine whether or not to treat.
It is even more difficult to diagnose active disease in the 1-4% of patients who remain serofast (CDC 2010. This means that the RPR pr VDRL test remains reactive. The only way to know if it is an infection that needs treatment is to compare titers. If the titer is four fold lower than pervious (i.e. 1:128 down to 1:32), this means that the person has been appropriately treated and no further therapy is needed (CDC, 2010). If the titer has risen, did not change or only decreased two fold (1:128 to 1:64), then the person has not had appropriate treatment or has been reinvented. Repeat testing should be done with the same test (either an RPR or VDRL) and ideally done by the same lab. RPR titers are often higher than VDRL titers.
Although there have been reports of unusually high or low or changing titers in HIV-infected persons, tests should be performed and interpreted in the same manner for HIV-infected individuals as for non-HIV-infected individuals (CDC 2010).
Testing for Neurosyphilis
The diagnosis of neurosyphilis is less direct and even more challenging than the diagnosis of other forms of syphilis. In 30% of cases of primary and secondary syphilis, T. pallidum does invade the cerebrospinal fluid but there is no obvious clinical signs or symptoms and no long term sequel. Therefore, it is not recommended to test for neurosyphilis routinely in individuals with primary and secondary syphilis.
However, some individuals, especially AIDS patients and other immunocompromised individuals, develop neurosyphilis. It is important to make this diagnosis because it can cause significant morbidity and mortality and it needs to be treated differently than other forms of syphilis. The antibiotics need to be given in high enough doses so that they penetrate the central nervous system.
Besides testing for nontreponemal and treponemal antibodies, persons with signs and symptoms of neurosyphilis, those who do not respond to treatment, and those with tertiary syphilis manifested by heart disease or gummas need to have lumbar puncture. A lumbar puncture is when a needle is inserted between the bones in the lumbar spine to remove cerebrospinal fluid (CSF), the fluid surrounding the brain (Mayo Clinc Staff, 2015). Certain tests need to be performed on the CSF. These include a VDRL, one of the nontreponemal antibody tests; a cell count to determine how many and what type of white blood cells; and protein level. The VDRL is highly specific but is not very sensitive so many individuals that have neurosyphilis do not have a positive CSF-VDRL. It the CSF-VDRL is negative, a white blood cell count >5 cells/mm3 or a protein level ≥ 46 mg/dl are suggestive of neurosyphilis (CDC, 2013). However, these tests are not specific, especially in HIV patients, who often have >5 white blood cell count /mm3 in the CDF (CDC, 2010).
Testing for Congenital Syphilis
It is recommended that all pregnant women should be tested for syphilis at the first prenatal visit and then repeated again during the third trimester (CDC, 2012). High risk women or those living in area with a high prevalence of syphilis should be tested again in third trimester and a delivery. Testing of the mother is done with nontreponemal and treponemal antibody tests as in other non-pregnant adults.
If syphilis is diagnosed during the second half of pregnancy in the mother, than the infant should immediately have an ultrasound to look for signs of syphilis such as live enlargement, fluid in body compartments, or a thickened placenta (CDC, 2010). If an infant is born to a mother with a reactive nontreponemal and treponemal, then the infant needs to be evaluated for congenital syphilis (CDC, 2014). The infant’s serum should be tested with a nontreponemal test but the infant’s blood may not be reactive especially if the mother has low titers or the mother was recently infected (CDC, 2010). The infant should also be examined for evidence of syphilis. Any skin lesions that are suspicious should be swabbed. Swabs from these lesions and other body fluids, and tissue from birth such as the placenta or umbilical cord should be examined under a darkfield microscope for T. pallidum. These specimens can also be examined for IgM specific antibodies using ELISA, Relispot, FTA-abs, or immunoblotting/Western blot. Specimens should be sent to the CDC for PCR (CDC, 2010). A lumbar puncture can also help establish the diagnosis. The same tests (VDRL, cell count and protein) are done as in adults who may have neurosyphilis. X-rays of the long bones and an ultrasound to look for abnormal fluid are also helpful.
Conclusion
Because of missed primary lesions, misdiagnosed secondary and tertiary signs and symptoms, lack of signs and symptoms during latent syphilis, and our inability to grow T. pallidum in the laboratory, syphilis can be challenging to diagnose. Direct testing, even with PCR has not provided much improvement in diagnosis. However, there are new point-of- care tests that will increase the availability and speed of antibody testing so that treatment can be given to more individuals with syphilis in the hope of slowing down or even halting this epidemic.
References
1. Causer LM, et al. An Evaluation of a Novel Dual Treponemal/Nontreponemal Point-of-Care Test for Syphilis as a Tool to Distinguish Active From Past Treated Infection. Clin Infect Dis. 2015 Mar 25. pii: civ243.
2. CDC. Sexually Transmitted Treatment Guidelines 2010. MMWR.2010;59:1-110.
3. CDC. Submitting Specimens to the CDC. http://www.cdc.gov/laboratory/specimen-submission/detail.html?CDCTestCode=CDC-10176. Last updated Arpil 13, 2015.
4. CDC. Syphilis CDC Fact Sheet http://www.cdc.gov/std/syphilis/stdfact-syphilis-detailed.htm. Last updated July 8, 2014.
5. CDC. Syphilis testing algorithms using treponemal tests for initial screening--four laboratories, New York City, 2005-2006.MMWR Morb Mortal Wkly Rep. 2008;57:872
6. CDC. Syphilis Curriculum. http://www2a.cdc.gov/stdtraining/ready-to-use/syphilis.htm. Lasted updated August 2013.
7. Czerkinsky CC, et al.Reverse enzyme-linked immunospot assay (RELISPOT) for the detection of cells secreting immunoreactive substances. J Immunol Methods. 1984;72:489-96.
8. Diagnostics Direct. 2011. Keep diagnostic tests & revenue in your office. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426843.htm
9. Fraser CM, et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375-88.
10. Genest, et al. Hum Pathol. 1996;27:366-72.
11. Grange PA, et al. Evaluation of a PCR test for detection of Treponema pallidum in swabs and blood. J Clin Microbiol. 2012;50:546-52.
12. Hagedorn HJ, et al. Evaluation of INNO-LIA syphilis assay as a confirmatory test for syphilis. J Clin Microbiol 2002;40:973-8.
13. Herring A, et al. WHO/TDR Sexually Transmitted Diseases Diagnostics Initiative. Evaluation of rapid diagnostic tests: syphilis. Nat Rev Microbiol. 2006; 4:S33-40.
14. Heymans R, et al. Clinical value of Treponema pallidum real-time PCR for diagnosis of syphilis. J Clin Microbiol. 2010;48:497-502.
15. Lab Tests Online. 2013. Syphilis Tests. http://labtestsonline.org/understanding/analytes/syphilis/tab/test/
16. Mayo Clinic Staff. 2015. Lumbar Puncture (spinal tap). http://www.mayoclinic.org/tests-procedures/lumbar-puncture/basics/definition/prc-20012679
17. Peeling RW, Hook EW 3rd. The pathogenesis of syphilis: the great mimicker, revisited. J Pathol 2006;208:224-32, 2014).
18. Shahrook S, Mori R, Ochirbat T, Gomi H. Strategies of testing for syphilis during pregnancy. Cochrane Database Syst Rev. 2014;10:CD010385. doi: 10.1002/14651858.CD010385.pub2.
19. Slonczewski, J L and Foster JW. Microbiology An Evolving Science. 3rd ed. New York: W. W. Norton & Company 2014.
20. Stoll BJ, et al. Clinical and serologic evaluation of neonates for congenital syphilis: a continuing diagnostic dilemma. . J Infect Dis. 1993;167:1093-9.
21. Tabidze IL, et al. Enzyme-linked immunospot assay for the diagnosis of active Treponema pallidum infection during the various stages of syphilis. Sex Transm Dis. 1999;26:426-30.
22. Tramont EC. Syphilis in adults: from Christopher Columbus to Sir Alexander Fleming to AIDS. Clin Infect Dis. 1995;21:1361-71.
23. Tramont EC. Treponema pallidum (syphilis). In: Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. 6th ed. New York: Churchill Livingston 2005:2768-85.
24. US Food and Drug Administration. 2014. FDA news Release. FDA grants CLIA waiver expanding the availability of rapid screening test for syphilis. http://www.fda.gov/NewsEvents/Newsroom/Pre
25. WHO. 2008. Global incidence an prevalence of selected curable sexually transmitted infections. http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf
26. Wikipedia. ELISPOT. http://en.wikipedia.org/wiki/ELISPOT. Updated Dec. 4, 2014
27. Yin YP, et al. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: a multisite evaluation study in China. Clin Infect Dis. 2013;56:659-65.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2015, Kenyon College.
