Rhodococcus ruber: Difference between revisions
| (72 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
==Classification== | ==Classification== | ||
[[ File:Scanning-electron-microscope-SEM-photomicrograph-of-a-four-day-old-biofilm-of-R-ruber.png | thumb| 250px| left| Figure 1: A ''Rhodococcus ruber'' biofilm on polystyrene [10] ]] | |||
Domain: Bacteria | Domain: Bacteria | ||
| Line 25: | Line 27: | ||
==Description and Significance== | ==Description and Significance== | ||
[[ File:DSM_44190.jpg | thumb| 200px| right| Figure | [[ File:DSM_44190.jpg | thumb| 200px| right| Figure 2: Glossy, orange colonies of ''Rhodococcus ruber'' cultured on a trypticase soy broth agar [https://bacdive.dsmz.de/strain/10942] ]] | ||
Historically, the genus ''Rhodococcus'' was first defined by Zopf in 1891. ''Nocardia rubra'', or what is known today as ''Rhodococcus ruber'', was isolated from a soil sludge by Kruse in 1896. However, there were issues with | Historically, the genus ''Rhodococcus'' was first defined by William Zopf in 1891. ''Nocardia rubra'', or what is known today as ''Rhodococcus ruber'', was isolated from a soil sludge by William Kruse in 1896. However, there were issues with taxonomic classifications due to inaccurate morphological and staining identification techniques. In 1977, Micheal Goodfellow and Grace Alderson did a complete reclassification of the genus ''Rhodococcus'', which resulted in ''Norcordia rubra'' being amended to ''Rhodococcus ruber'' [1,2]. | ||
The term ''Rhodococcus'' is from the Greek words “rhodon” and “coccus” meaning “rose” and “grain,“ respectively. ''R. ruber's'' morphological structure matches the origins of its name: rods and cocci [1]. ''R. ruber'' initially forms a branching mycelium that breaks into shorter rods and cocci as it transitions through different growth phases. ''R. ruber'' is characteristically a gram positive, non-motile, non-spore forming bacteria [4,7]. It has diverse metabolic and nutritional capabilities depending on the strain, such as utilizing gaseous hydrocarbons, aromatic hydrocarbons, complex polymers, and steroids for carbon and energy sources [1,4,7]. | |||
''R. ruber'' is unique in that it is ubiquitous in many different habitats whether that is pristine, contaminated, temperate, or extreme. It is able to persist and tolerate stressful conditions, such as environments with limited oxygen and/or nutrients [1,4,7]. Given its metabolic and habitat versatility, ''R. ruber'' is a good prospect for bioremediation and other biotechnological uses to naturally degrade pollutants and remedy contaminated ecosystems [1,4,6]. | |||
==Genome Structure== | ==Genome Structure== | ||
12 genome studies have been reported for the following strains of ''Rhodococcus ruber'': ''R. ruber str. YC-YT1'', ''R. ruber str. YYL'', ''R. ruber str. P14'', ''R. ruber str. SD3'', ''R. ruber str. R1'', ''R. ruber str. DSM 43338'', ''R. ruber str. P25'', ''R. tuber IEGM 231'', ''R. ruber str. OA1'', ''R. ruber str. BKS 20-38'', ''R. ruber str. Chol-4'', and ''R. ruber str. NCRX 15591''. | 12 genome studies have been reported for the following strains of ''Rhodococcus ruber'': ''R. ruber str. YC-YT1'', ''R. ruber str. YYL'', ''R. ruber str. P14'', ''R. ruber str. SD3'', ''R. ruber str. R1'', ''R. ruber str. DSM 43338'', ''R. ruber str. P25'', ''R. tuber IEGM 231'', ''R. ruber str. OA1'', ''R. ruber str. BKS 20-38'', ''R. ruber str. Chol-4'', and ''R. ruber str. NCRX 15591'' [11]. | ||
The genome of ''Rhodococcus ruber'' consists of one circular chromosome with an average size of 5.57 Mb. The genome size varies for every strain and larger genome sizes can be attributed to the presence of circular plasmids. The strains of ''R. ruber'' that contain plasmids are YC-TC1, YYL, and R1. The average amount of protein coding genes is 4932 and the average G+C content is 70.49%. A high guanine-cytosine content is characteristic of ''Rhodococcus'' species | The genome of ''Rhodococcus ruber'' consists of one circular chromosome with an average size of 5.57 Mb [3,11]. The genome size varies for every strain and larger genome sizes can be attributed to the presence of linear or circular plasmids [1,6]. The strains of ''R. ruber'' that contain plasmids are YC-TC1, YYL, and R1, which are known to contain additional genes that code metabolic enzymes [6,11]. The average amount of protein coding genes is 4932 and the average G+C content is 70.49% [11]. A high guanine-cytosine content is characteristic of ''Rhodococcus'' species which provides DNA stability in varying conditions [1]. | ||
The genome sequences of ''R. ruber'' are helpful in identifying the genes necessary for metabolic pathways and cycles that ''R. ruber'' employs including the tricarboxylic acid cycle, pentose phosphate pathway, glycolysis, and | The genome sequences of ''R. ruber'' are helpful in identifying the genes necessary for metabolic pathways and cycles that ''R. ruber'' employs including the tricarboxylic acid cycle, pentose phosphate pathway, glycolysis, and gluconeogenesis [2]. Additionally, the beta oxidation pathway and alkane degradation pathway are utilized to break down more complex xenobiotic compounds before the classic metabolic pathways are used [5]. The genome highlights multiple metabolic gene clusters that are used for degradation of aromatic compounds, steroids, hydrocarbons, and xenobiotic polymers that ''R. ruber'' uses for carbon and energy sources. Some strains of ''R. ruber'' contain redundant genes which contribute to their versatile metabolic abilities with various nutrient sources [6]. | ||
==Cell Structure and Metabolism== | ==Cell Structure and Metabolism== | ||
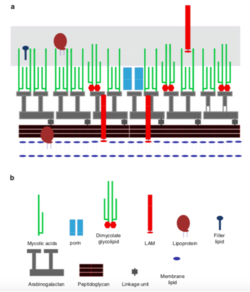
[[ File:Screen Shot 2020-04-29 at 4.19.14 AM.png| thumb| | [[ File:Screen Shot 2020-04-29 at 4.19.14 AM.png| thumb| 250px| left| Figure 3: ''Rhodococcus ruber'' cell wall structure [1] ]] | ||
''Rhodococcus ruber'' is a gram positive bacteria that is non-motile and non-spore forming. Key features of its cell structure include its thick peptidoglycan layer, mycolic acids, lipoglycans, and cell envelope lipids [1,4,6]. The mycolic acids of ''R. ruber'' are long chain fatty acids with chain lengths ranging from C42-C48, which are much longer than the carbon chains in other ''Rhodococcus'' species [4,6]. Its mycolic acids are composed of saturated and unsaturated fatty acids with tuberculostearic acid [1,6]. As with all ''Rhodococcus'' species, its cell wall is made up of the carbohydrates arabinose and galactose [1,4,6]. ''R. ruber's'' lipoglycans are polysaccharides in the lipoarabinomannan family that are anchored into the membrane [1]. The cell envelope lipids of ''R. ruber'' have notable surfactant properties, allowing ''R. ruber'' to attach and grow on hydrophobic substrates. Other biomolecules that contribute to ''R. ruber's'' hydrophobicity and substrate binding includes its mycolic acids, extracellular polymeric substance, and cell surface proteins [1,8]. | |||
''R. ruber'' is an aerobic chemoorganotroph, meaning they use organic compounds as their carbon and energy sources through oxidative metabolic pathways [1]. It is able to utilize gaseous hydrocarbons, aliphatic hydrocarbons, aromatic hydrocarbons, and xenobiotic substances, such as crude oil and plastics [1,4,5,6,10,13]. Additionally, certain strains of ''R. ruber'' have unique metabolic abilities, such as ''R. ruber str. Chol-4'' and ''R. ruber str. C208.'' ''R. ruber str. Chol-4'' is able to use steroids, such as cholesterol, testosterone, and phytosterols, as their carbon source. In the interest of biotechnological applications, the metabolic activity of ''R. ruber str. Chol-4'' can be used for steroid transformations to active pharmaceuticals [6]. The other strain with unique metabolic abilities is ''R. ruber str. C208.'' This strain is able to use inert polymers, such as polyethylene and polystyrene, as its sole carbon source [5,9,10,13]. ''R. ruber str. C208'' utilizes beta oxidation and alkane degradation pathways to break down these polymers [5]. Additionally, it has laccase-coding genes that promote biotic oxidation. Laccases are phenol oxidases that belong to a group of multi-copper enzymes. Studies have shown that the addition of copper in polyethylene cultures improved laccase's oxidizing activity, thereby resulting in increased degradation [9,12]. ''R. ruber str. C208'' has important implications regarding bioremediation efforts. | |||
==Environmental Impacts== | |||
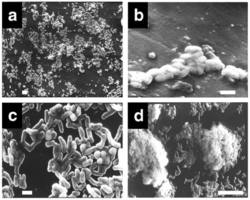
[[ File:Screen Shot 2020-04-29 at 5.07.24 AM.png| thumb| 250px| right| Figure 4: Biofilm formation of ''Rhodococcus ruber str. C208'' on polyethylene. A) Attachment to polyethylene surface B) Formation of monolayer and extracellular polymeric substance C) Formation of a multilayer microcolony D) Mature biofilm in its characteristic "mushroom" shape [13] ]] | |||
Environmental pollution of plastics and organic compounds are becoming an increasing threat to global biodiversity, natural ecological functions, and global food production [1,9]. Approximately over 300 million tons of plastics are produced annually and the non-biodegradable polymer, polyethylene, makes up up half of that amount [9]. The main source of anthropogenic hydrocarbons are from oil spills and industrial waste deposits [1]. Techniques such as recycling practices and oil spill cleanups are not enough to clear the harmful waste products everywhere. Because of this, a more sustainable method is necessary for future remediation and disposal methods [1,9]. Given ''Rhodococcus ruber's'' metabolic diversity to various organic and xenobiotic compounds, it can be a key player in bioremediation efforts to accelerate the degradation of environmental contaminants. | |||
[[ | With polymers like polyethylene and polystyrene, ''R. ruber'' will form a biofilm [9,10,13]. In an environment starved of carbon source, ''R. ruber'' will colonize the surface and reproduce. The polymer acts a substrate and carbon source for ''R. ruber,'' allowing it to have greater metabolic activity when it is in a biofilm formation [10,13]. It is able to adhere to the surface through hydrophobic interactions and surfactant properties associated with its cellular structure. After a monolayer is formed, ''R. ruber'' continues to proliferate and form multicellular structures that are long lasting and enhance the rate of biodegradation of polymers like polyethylene and polystyrene [9,10,13]. | ||
With hydrocarbon pollutants, like crude oil and petroleum, ''R. ruber'' is able to use these as carbon and energy sources. It is commonly associated with oil-bearing sites due to its hydrophobic cell surface, and thus, is able to emulsify oil from water sources. This allows it to have profound impacts in oil prospecting and bioremediation efforts through the identification and breakdown of these environmental pollutants. Small-scale field studies have shown the positive effects of ''R. ruber'' in contaminated soil and aquatic environments with modified ''R. ruber'' treatments, such as liquid biopreparations and biofertilizers [1,9]. | |||
==References== | ==References== | ||
[1][https://www.springer.com/gp/book/9783030114602 | Alvarez, H. M. Biology of Rhodococcus; Springer, 2010; Vol. 16.] | [1][https://www.springer.com/gp/book/9783030114602 | Alvarez, H. M. Biology of Rhodococcus; Springer, 2010; Vol. 16.] | ||
[2] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623001/ | Bala] | [2] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623001/ | Bala, M.; Kumar, S.; Raghava, G. P. S.; Mayilraj, S. Draft Genome Sequence of Rhodococcus ruber Strain BKS 20-38. Genome Announcements 2013, 1.] | ||
[3] [https://mra.asm.org/content/9/2/e00905-19 | Farkas] | [3] [https://mra.asm.org/content/9/2/e00905-19 | Farkas, C.; Donoso, R. A.; Melis-Arcos, F.; Gárate-Castro, C.; Pérez-Pantoja, D. Complete Genome Sequence of Rhodococcus ruber R1, a Novel Strain Showing a Broad Catabolic Potential toward Lignin-Derived Aromatics. Microbiology Resource Announcements 2020, 9.] | ||
[4] [https://www.ncbi.nlm.nih.gov/pubmed/1444254 | Finnerty, W. R. The Biology and Genetics of the Genus Rhodococcus. Annual Review of Microbiology 1992, 46, 193–218.] | [4] [https://www.ncbi.nlm.nih.gov/pubmed/1444254 | Finnerty, W. R. The Biology and Genetics of the Genus Rhodococcus. Annual Review of Microbiology 1992, 46, 193–218.] | ||
| Line 71: | Line 68: | ||
[5] [https://pubs-acs-org.proxyiub.uits.iu.edu/doi/pdf/10.1021/acs.est.7b00846 | Gravouil, K.; Ferru-Clément, R.; Colas, S.; Helye, R.; Kadri, L.; Bourdeau, L.; Moumen, B.; Mercier, A.; Ferreira, T. Transcriptomics and Lipidomics of the Environmental Strain Rhodococcus ruber Point out Consumption Pathways and Potential Metabolic Bottlenecks for Polyethylene Degradation. Environmental Science & Technology 2017, 51, 5172–5181.] | [5] [https://pubs-acs-org.proxyiub.uits.iu.edu/doi/pdf/10.1021/acs.est.7b00846 | Gravouil, K.; Ferru-Clément, R.; Colas, S.; Helye, R.; Kadri, L.; Bourdeau, L.; Moumen, B.; Mercier, A.; Ferreira, T. Transcriptomics and Lipidomics of the Environmental Strain Rhodococcus ruber Point out Consumption Pathways and Potential Metabolic Bottlenecks for Polyethylene Degradation. Environmental Science & Technology 2017, 51, 5172–5181.] | ||
[6] [https://www.ncbi.nlm.nih.gov/pubmed/31046661 | Guevara] | [6] [https://www.ncbi.nlm.nih.gov/pubmed/31046661 | Guevara, G.; Lopez, M. C.; Alonso, S.; Perera, J.; Navarro-Llorens, J. M. New insights into the genome of Rhodococcus ruber strain Chol-4. BMC Genomics 2019, 20.] | ||
[7] [https://www.ncbi.nlm.nih.gov/pubmed/19688376 | Heras, L. F. D. L.; Fernández, E. G.; Llorens, J. M. N.; Perera, J.; Drzyzga, O. Morphological, Physiological, and Molecular Characterization of a Newly Isolated Steroid-Degrading Actinomycete, Identified as Rhodococcus ruber Strain Chol-4. Current Microbiology 2009, 59, 548–553.] | [7] [https://www.ncbi.nlm.nih.gov/pubmed/19688376 | Heras, L. F. D. L.; Fernández, E. G.; Llorens, J. M. N.; Perera, J.; Drzyzga, O. Morphological, Physiological, and Molecular Characterization of a Newly Isolated Steroid-Degrading Actinomycete, Identified as Rhodococcus ruber Strain Chol-4. Current Microbiology 2009, 59, 548–553.] | ||
[8] [https://www.mdpi.com/2073-4344/9/3/236/htm | | [8] [https://www.mdpi.com/2073-4344/9/3/236/htm | Krivoruchko, A.; Kuyukina, M.; Ivshina, I. Advanced Rhodococcus Biocatalysts for Environmental Biotechnologies. Catalysts 2019, 9.] | ||
[9] [https://www.mdpi.com/2073-4360/12/1/123/htm | Montazer, Z.; Najafi, M. B. H.; Levin, D. B. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers 2020, 12.] | [9] [https://www.mdpi.com/2073-4360/12/1/123/htm | Montazer, Z.; Najafi, M. B. H.; Levin, D. B. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers 2020, 12.] | ||
| Line 81: | Line 78: | ||
[10] [https://www.ncbi.nlm.nih.gov/pubmed/18401686 | Mor, R.; Sivan, A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber. Biodegradation 2008, 19, 851–858.] | [10] [https://www.ncbi.nlm.nih.gov/pubmed/18401686 | Mor, R.; Sivan, A. Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber. Biodegradation 2008, 19, 851–858.] | ||
[11] | [11] Rhodococcus ruber (ID 11562) https://www.ncbi.nlm.nih.gov/genome/?term=txid1830[Organism:noexp] (accessed 2020). | ||
[12 | [12 [https://www.researchgate.net/publication/271892890_The_role_of_the_copper-binding_enzyme_-_laccase_-_in_the_biodegradation_of_polyethylene_by_the_actinomycete_Rhodococcus_ruber | Santo, M.; Weitsman, R.; Sivan, A. The role of the copper-binding enzyme – laccase – in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. International Biodeterioration & Biodegradation 2013, 84, 204–210.] | ||
[13] [https://www.ncbi.nlm.nih.gov/ | [13] [https://www.ncbi.nlm.nih.gov/pubmed/16534612 | Sivan, A.; Szanto, M.; Pavlov, V. Biofilm development of the polyethylene-degrading bacterium Rhodococcus ruber. Applied Microbiology and Biotechnology 2006, 72, 346–352.] | ||
==Author== | ==Author== | ||
Latest revision as of 01:41, 1 May 2020
Classification
Domain: Bacteria
Phylum: Actinobacteria
Class: Actinobacteria
Order: Corynbacteriales
Family: Nocardiaceae
Genus: Rhodococcus
Species
|
NCBI: [2] |
Rhodococcus ruber
Description and Significance
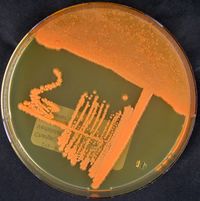
Historically, the genus Rhodococcus was first defined by William Zopf in 1891. Nocardia rubra, or what is known today as Rhodococcus ruber, was isolated from a soil sludge by William Kruse in 1896. However, there were issues with taxonomic classifications due to inaccurate morphological and staining identification techniques. In 1977, Micheal Goodfellow and Grace Alderson did a complete reclassification of the genus Rhodococcus, which resulted in Norcordia rubra being amended to Rhodococcus ruber [1,2].
The term Rhodococcus is from the Greek words “rhodon” and “coccus” meaning “rose” and “grain,“ respectively. R. ruber's morphological structure matches the origins of its name: rods and cocci [1]. R. ruber initially forms a branching mycelium that breaks into shorter rods and cocci as it transitions through different growth phases. R. ruber is characteristically a gram positive, non-motile, non-spore forming bacteria [4,7]. It has diverse metabolic and nutritional capabilities depending on the strain, such as utilizing gaseous hydrocarbons, aromatic hydrocarbons, complex polymers, and steroids for carbon and energy sources [1,4,7].
R. ruber is unique in that it is ubiquitous in many different habitats whether that is pristine, contaminated, temperate, or extreme. It is able to persist and tolerate stressful conditions, such as environments with limited oxygen and/or nutrients [1,4,7]. Given its metabolic and habitat versatility, R. ruber is a good prospect for bioremediation and other biotechnological uses to naturally degrade pollutants and remedy contaminated ecosystems [1,4,6].
Genome Structure
12 genome studies have been reported for the following strains of Rhodococcus ruber: R. ruber str. YC-YT1, R. ruber str. YYL, R. ruber str. P14, R. ruber str. SD3, R. ruber str. R1, R. ruber str. DSM 43338, R. ruber str. P25, R. tuber IEGM 231, R. ruber str. OA1, R. ruber str. BKS 20-38, R. ruber str. Chol-4, and R. ruber str. NCRX 15591 [11].
The genome of Rhodococcus ruber consists of one circular chromosome with an average size of 5.57 Mb [3,11]. The genome size varies for every strain and larger genome sizes can be attributed to the presence of linear or circular plasmids [1,6]. The strains of R. ruber that contain plasmids are YC-TC1, YYL, and R1, which are known to contain additional genes that code metabolic enzymes [6,11]. The average amount of protein coding genes is 4932 and the average G+C content is 70.49% [11]. A high guanine-cytosine content is characteristic of Rhodococcus species which provides DNA stability in varying conditions [1].
The genome sequences of R. ruber are helpful in identifying the genes necessary for metabolic pathways and cycles that R. ruber employs including the tricarboxylic acid cycle, pentose phosphate pathway, glycolysis, and gluconeogenesis [2]. Additionally, the beta oxidation pathway and alkane degradation pathway are utilized to break down more complex xenobiotic compounds before the classic metabolic pathways are used [5]. The genome highlights multiple metabolic gene clusters that are used for degradation of aromatic compounds, steroids, hydrocarbons, and xenobiotic polymers that R. ruber uses for carbon and energy sources. Some strains of R. ruber contain redundant genes which contribute to their versatile metabolic abilities with various nutrient sources [6].
Cell Structure and Metabolism
Rhodococcus ruber is a gram positive bacteria that is non-motile and non-spore forming. Key features of its cell structure include its thick peptidoglycan layer, mycolic acids, lipoglycans, and cell envelope lipids [1,4,6]. The mycolic acids of R. ruber are long chain fatty acids with chain lengths ranging from C42-C48, which are much longer than the carbon chains in other Rhodococcus species [4,6]. Its mycolic acids are composed of saturated and unsaturated fatty acids with tuberculostearic acid [1,6]. As with all Rhodococcus species, its cell wall is made up of the carbohydrates arabinose and galactose [1,4,6]. R. ruber's lipoglycans are polysaccharides in the lipoarabinomannan family that are anchored into the membrane [1]. The cell envelope lipids of R. ruber have notable surfactant properties, allowing R. ruber to attach and grow on hydrophobic substrates. Other biomolecules that contribute to R. ruber's hydrophobicity and substrate binding includes its mycolic acids, extracellular polymeric substance, and cell surface proteins [1,8].
R. ruber is an aerobic chemoorganotroph, meaning they use organic compounds as their carbon and energy sources through oxidative metabolic pathways [1]. It is able to utilize gaseous hydrocarbons, aliphatic hydrocarbons, aromatic hydrocarbons, and xenobiotic substances, such as crude oil and plastics [1,4,5,6,10,13]. Additionally, certain strains of R. ruber have unique metabolic abilities, such as R. ruber str. Chol-4 and R. ruber str. C208. R. ruber str. Chol-4 is able to use steroids, such as cholesterol, testosterone, and phytosterols, as their carbon source. In the interest of biotechnological applications, the metabolic activity of R. ruber str. Chol-4 can be used for steroid transformations to active pharmaceuticals [6]. The other strain with unique metabolic abilities is R. ruber str. C208. This strain is able to use inert polymers, such as polyethylene and polystyrene, as its sole carbon source [5,9,10,13]. R. ruber str. C208 utilizes beta oxidation and alkane degradation pathways to break down these polymers [5]. Additionally, it has laccase-coding genes that promote biotic oxidation. Laccases are phenol oxidases that belong to a group of multi-copper enzymes. Studies have shown that the addition of copper in polyethylene cultures improved laccase's oxidizing activity, thereby resulting in increased degradation [9,12]. R. ruber str. C208 has important implications regarding bioremediation efforts.
Environmental Impacts
Environmental pollution of plastics and organic compounds are becoming an increasing threat to global biodiversity, natural ecological functions, and global food production [1,9]. Approximately over 300 million tons of plastics are produced annually and the non-biodegradable polymer, polyethylene, makes up up half of that amount [9]. The main source of anthropogenic hydrocarbons are from oil spills and industrial waste deposits [1]. Techniques such as recycling practices and oil spill cleanups are not enough to clear the harmful waste products everywhere. Because of this, a more sustainable method is necessary for future remediation and disposal methods [1,9]. Given Rhodococcus ruber's metabolic diversity to various organic and xenobiotic compounds, it can be a key player in bioremediation efforts to accelerate the degradation of environmental contaminants.
With polymers like polyethylene and polystyrene, R. ruber will form a biofilm [9,10,13]. In an environment starved of carbon source, R. ruber will colonize the surface and reproduce. The polymer acts a substrate and carbon source for R. ruber, allowing it to have greater metabolic activity when it is in a biofilm formation [10,13]. It is able to adhere to the surface through hydrophobic interactions and surfactant properties associated with its cellular structure. After a monolayer is formed, R. ruber continues to proliferate and form multicellular structures that are long lasting and enhance the rate of biodegradation of polymers like polyethylene and polystyrene [9,10,13].
With hydrocarbon pollutants, like crude oil and petroleum, R. ruber is able to use these as carbon and energy sources. It is commonly associated with oil-bearing sites due to its hydrophobic cell surface, and thus, is able to emulsify oil from water sources. This allows it to have profound impacts in oil prospecting and bioremediation efforts through the identification and breakdown of these environmental pollutants. Small-scale field studies have shown the positive effects of R. ruber in contaminated soil and aquatic environments with modified R. ruber treatments, such as liquid biopreparations and biofertilizers [1,9].
References
[1]| Alvarez, H. M. Biology of Rhodococcus; Springer, 2010; Vol. 16.
[11] Rhodococcus ruber (ID 11562) https://www.ncbi.nlm.nih.gov/genome/?term=txid1830[Organism:noexp] (accessed 2020).
Author
Page authored by Hannah von Werder, student of Prof. Jay Lennon at Indiana University.