Bacteroides fragilis: Commensal yet Pathogenic: Difference between revisions
| (24 intermediate revisions by the same user not shown) | |||
| Line 12: | Line 12: | ||
[[Image:Fragpiechart.png|thumb|230px|right|Figure 2. Clinical proportions of species found within the <i>Bacteroides</i> genus. By Hannah M. Wexler, Wadsworth Anaerobe Library. [https://pubmed.ncbi.nlm.nih.gov/17934076/].]]<i>Bacteroides fragilis</i> is a gram-negative, non-spore forming, rod-like anaerobic microbe.<ref name = nitty>Wexler HM. <i>Bacteroides: the good, the bad, and the nitty-gritty.</i> Clin Microbiol Rev. 2007 Oct;20(4):593-621.</ref> The bacteria reside in the gut flora of the human microbiota. <i>Bacteroides fragilis</i> remain commensal with the host when in the gut. There, they play an essential role in processing complex molecules into simpler ones in the host intestines. However, if the microbe enters the bloodstream or surrounding tissue it can lead to severe anaerobic infections, most commonly the formation of abscesses. | [[Image:Fragpiechart.png|thumb|230px|right|Figure 2. Clinical proportions of species found within the <i>Bacteroides</i> genus. By Hannah M. Wexler, Wadsworth Anaerobe Library. [https://pubmed.ncbi.nlm.nih.gov/17934076/].]]<i>Bacteroides fragilis</i> is a gram-negative, non-spore forming, rod-like anaerobic microbe.<ref name = nitty>Wexler HM. <i>Bacteroides: the good, the bad, and the nitty-gritty.</i> Clin Microbiol Rev. 2007 Oct;20(4):593-621.</ref> The bacteria reside in the gut flora of the human microbiota. <i>Bacteroides fragilis</i> remain commensal with the host when in the gut. There, they play an essential role in processing complex molecules into simpler ones in the host intestines. However, if the microbe enters the bloodstream or surrounding tissue it can lead to severe anaerobic infections, most commonly the formation of abscesses. | ||
Anaerobes are the most common bacteria in the human colon. Within the group of anaerobes, <i>Bacteroides</i> make up approximately 25% of the anaerobic bacteria within the gut.<ref> Salyers, A. A. 1984. <i>Bacteroides of the human lower intestinal tract.</i> Annu. Rev. Microbial. 38:293-313.</ref> The <i>Bacteroides</i> genus consists of: <i>B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B. vulgatus, B. uniformis, B. eggerthii, B. merdae, B. stercoris, and B. caccae</i> (shown in Figure 2). Of these, <i>B. fragilis</i> is the most frequent isolate and responsible for the majority of | Anaerobes are the most common bacteria in the human colon. Within the group of anaerobes, <i>Bacteroides</i> make up approximately 25% of the anaerobic bacteria within the gut.<ref> Salyers, A. A. 1984. <i>Bacteroides of the human lower intestinal tract.</i> Annu. Rev. Microbial. 38:293-313.</ref> The <i>Bacteroides</i> genus consists of: <i>B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B. vulgatus, B. uniformis, B. eggerthii, B. merdae, B. stercoris, and B. caccae</i> (shown in Figure 2). Of these, <i>B. fragilis</i> is the most frequent isolate and responsible for the majority of anaerobic infections.<ref name = nitty /> <i>B. fragilis</i> is most commonly associated with intra-abdominal infections.<ref>Goldstein, E. J. 1996. <i>Anaerobic bactermia</i> Clin. Infect. Dis. 23:97-101.</ref> A number of these species demonstrate resistance to a wide variety of current antibiotics. | ||
<br><br> | |||
[[Image:efflux.png|thumb| | [[Image:efflux.png|thumb|315px|left|Figure 3. A model of the <i>Bacteroides fragilis</i> cell envelope. By Pumbwe et al., Department of Medicine, UCLA. [https://pubmed.ncbi.nlm.nih.gov/17045496/].]] | ||
==Cellular Structure== | ==Cellular Structure== | ||
Elements of the cellular structure of <i>B. fragilis</i> contribute to its commensal relationship to the host or its advantages as a pathogen. Like all species of the <i>Bacteroides</i> genus, <i>B. fragilis</i> is a rod-like anaerobe with an inner cell membrane and an outer cell membrane containing lipopolysaccharides with specific side chains.<ref name = nitty /> These side chains | Elements of the cellular structure of <i>B. fragilis</i> contribute to its commensal relationship to the host or its advantages as a pathogen. Like all species of the <i>Bacteroides</i> genus, <i>B. fragilis</i> is a rod-like anaerobe with an inner cell membrane and an outer cell membrane containing lipopolysaccharides with specific side chains.<ref name = nitty /> These side chains (polysaccharide A and polysaccharide B) are made up of repeating tetrasaccharide and hexasaccharide units, respectively(shown in Figure 3, labeled A and B). These polysaccharides consist of positively and negatively charged groups that interacted with each other to form a capsule that can be up to several hundred micrometers thick.<ref name = Pumbwe /> The polysaccharide complex is responsible for induction of abscess formation when the bacterium escapes the gut. There are also pili and efflux pumps protruding through the outer cell membrane(labeled EP and Pi). The pili are responsible for adherence to surrounding cells and mucus and the efflux pumps play a role in excreting toxins and antibiotics, contributing to the high level antibiotic resistance of <i>B. fragilis</i>.<ref>Pumbwe L, Ueda O, Yoshimura F, Chang A, Smith R, Wexler HM. <i>Bacteroides fragilis BmeABC efflux systems additively confer intrinsic antimicrobial resistance.</i> J Antimicrob Chemother 2006; 58:37-46.</ref> <i>B. fragilis</i> also contain a large variation of proteins and enzymes that it uses to obtain and digest the wide variety of carbohydrates available in the gut. This is ideal for growth within the ever changing environment of the large intestine. This complex array of enzymes benefits the microbiome by breaking down nutrients for the other surrounding gut bacteria that are unable to digest the nutrients themselves. <i>B. fragilis</i> also produces a bile salt hydrolase to protect the anaerobe from the bile salts within in the gut. Without this enzyme, the bile salts would collapse its membrane and damage the cell.<ref name = Pumbwe/> <i>B. fragilis</i> also has the ability to adapt its surface architecture to the conditions within the gut or outside the gut. This function allows it to survive the changing and harsh conditions of the gut or evade the host immune system outside the gut. This complex cell surface structure allows for its dual existence as a commensal gut bacteria and, when escaped, a pathogen. | ||
<br><br> | <br><br> | ||
==Pathogenesis and Epidemiology== | ==Pathogenesis and Epidemiology== | ||
<i>B. fragilis</i> only makes up about 1-2% of the gut micro-flora, yet it is the most predominant and persistent isolate found at the site of anaerobic infection.<ref name = Pumbwe>Pumbwe L, Skilbeck CA, Wexler HM. <i>The Bacteroides fragilis cell envelope: quarterback, linebacker, coach-or all three?</i> Anaerobe. 2006 Oct-Dec;12(5-6):211-20.</ref> Infections from <i>B. fragilis</i> commonly arise from a break in the mucosal membrane of the intestine.<ref>Elsaghir H, Reddivari AKR. <i>Bacteroides Fragilis.</i> [Updated 2020 Jun 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-.</ref> The travel of <i>B. fragilis</i> outside of the gut can lead to a variety of infections such as local abscesses at the site of the | <i>B. fragilis</i> only makes up about 1-2% of the gut micro-flora, yet it is the most predominant and persistent isolate found at the site of anaerobic infection.<ref name = Pumbwe>Pumbwe L, Skilbeck CA, Wexler HM. <i>The Bacteroides fragilis cell envelope: quarterback, linebacker, coach-or all three?</i> Anaerobe. 2006 Oct-Dec;12(5-6):211-20.</ref> Infections from <i>B. fragilis</i> commonly arise from a break in the mucosal membrane of the intestine.<ref>Elsaghir H, Reddivari AKR. <i>Bacteroides Fragilis.</i> [Updated 2020 Jun 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-.</ref> The travel of <i>B. fragilis</i> outside of the gut can lead to a variety of infections such as local abscesses at the site of the mucosal disruption, metastatic abscesses by spread through the bloodstream to distant organs such as the abdomen, brain, pelvis, or liver, or abscesses formed in the lungs by aspiration of the microbe.<ref name = Pumbwe/> | ||
<i>Bacteroides</i> species can be passed from a mother to offspring during childbirth, integrating itself into the human flora.<ref>Reid G. 2004. <i>When microbe meets human.</i> Clin. Infect. Dis.39:827-830.</ref> Species of <i>Bacteroides</i>, including <i>B. fragilis</i>, appear in the newborn microbiota about 10 days after birth.<ref>Simon, G. L., and S. L. Gorbach. 1984. <i>Intestinal flora in health and disease.</i> Gastroenterology 86:174-193.</ref> Predisposition to <i>B. fragilis</i> infection can arise from surgery, disease, or trauma which can cause perforation of the gastrointestinal tract leading to pathogenesis. | <i>Bacteroides</i> species can be passed from a mother to offspring during childbirth, integrating itself into the human flora.<ref>Reid G. 2004. <i>When microbe meets human.</i> Clin. Infect. Dis.39:827-830.</ref> Species of <i>Bacteroides</i>, including <i>B. fragilis</i>, appear in the newborn microbiota about 10 days after birth.<ref>Simon, G. L., and S. L. Gorbach. 1984. <i>Intestinal flora in health and disease.</i> Gastroenterology 86:174-193.</ref> Predisposition to <i>B. fragilis</i> infection can arise from surgery, disease, or trauma which can cause perforation of the gastrointestinal tract leading to pathogenesis.<ref name = nitty /> The polysaccharide capsule created by the outer membrane of the cell contributes to the induction of abscess formation. When the host recognizes this polysaccharide complex, it triggers the formation of a fibrous wall that encapsulates the anaerobic colony.<ref name = Pumbwe/> Because the <i>B. fragilis</i> is anaerobic, it thrives without oxygen. Normally, an aerobic bacterial infection would be suffocated by the fibrous wall due to lack of oxygen; however, the anaerobic <i>B. fragilis</i> continues to grow and expand. The polysaccharide capsule combined with the anaerobic nature of the bacterium contributes to its powerful success as a pathogen. | ||
When the host recognizes this polysaccharide complex, it | |||
<i>E. coli</i>, a facultative anaerobe, uses the oxygen at the site which brings the oxygen to a level where anaerobes, such as <i>B. fragilis</i>, can thrive and grow. Due to this phenomenon, many severe anaerobic infections contain a mix of anaerobic and aerobic flora. | <i>E. coli</i>, a facultative anaerobe, uses the oxygen at the site which brings the oxygen to a level where anaerobes, such as <i>B. fragilis</i>, can thrive and grow. Due to this phenomenon, many severe anaerobic infections contain a mix of anaerobic and aerobic flora. | ||
| Line 39: | Line 38: | ||
==Virulence== | ==Virulence== | ||
<b>Adherence to Tissues:</b> The fimbriae (shown in | <b>Adherence to Tissues:</b> The fimbriae(multiple pili) (shown in Figure 4 at left) of <i>B. fragilis</i> allows for easy adhesion to epithelial cells and extracellular molecules. <i>B. fragilis</i> can also adhere to peritoneal surfaces due to its unique polysaccharide capsules, a structural function that not many other anaerobes possess. These adhesive properties permit <i>B. fragilis</i> to establish itself within the host tissue and avoid immune targeting and attack.<ref name = nitty /> | ||
<b>Protection from Host:</b> The ability of a bacterium to protect itself from the immune response of its host is a large contributing factor to the species' virulence. Due to its polysaccharide capsule, a key promoter in the formation of intraabdominal abscesses, its LPS (Lipopolysaccharide), the main component of the outer membrane of gram-negative bacteria, and a variety of protective enzymes <i>B. fragilis</i> is able to defend itself from the immune response of the host.<ref name = nitty /><ref>Mancuso, Giuseppe et al. <i>Bacteroides fragilis-derived lipopolysaccharide produces cell activation and lethal toxicity via toll-like receptor 4.</i> Infection and immunity vol. 73,9 (2005): 5620-7.</ref> | <b>Protection from Host:</b> The ability of a bacterium to protect itself from the immune response of its host is a large contributing factor to the species' virulence. Due to its polysaccharide capsule, a key promoter in the formation of intraabdominal abscesses, its LPS (Lipopolysaccharide), the main component of the outer membrane of gram-negative bacteria, and a variety of protective enzymes <i>B. fragilis</i> is able to defend itself from the immune response of the host.<ref name = nitty /><ref>Mancuso, Giuseppe et al. <i>Bacteroides fragilis-derived lipopolysaccharide produces cell activation and lethal toxicity via toll-like receptor 4.</i> Infection and immunity vol. 73,9 (2005): 5620-7.</ref> | ||
<b>Destruction of Tissues:</b> Histolytic enzymes of <i>B. fragilis</i> control and induce tissue destruction. The histolytic enzymes attack the extracellular matrix of the host, allowing for the infection to expand into this space.<ref>Rudek, W. R. U. Haque. 1976. <i>Extracellular enzymes of the genus Bacteroides.</i> J. Clin. Microbiol. 4:458-460.</ref> This destruction of the host is one of the main reasons why <i>B. fragilis</i> is considered so virulent and dangerous. As the abscesses form, the <i>B. fragilis</i> also can eliminate surrounding tissue often leading to permanent damage. Abscesses that remain untreated for prolonged periods of time can expand in size and even lead to intestinal obstruction or blood vessel invasion.<ref name = nitty /> | <b>Destruction of Tissues:</b> Histolytic enzymes of <i>B. fragilis</i> control and induce tissue destruction. The histolytic enzymes attack the extracellular matrix of the host, allowing for the infection to expand into this space.<ref>Rudek, W. R. U. Haque. 1976. <i>Extracellular enzymes of the genus Bacteroides.</i> J. Clin. Microbiol. 4:458-460.</ref> This destruction of the host is one of the main reasons why <i>B. fragilis</i> is considered so virulent and dangerous. As the abscesses form, the <i>B. fragilis</i> also can eliminate surrounding tissue often leading to permanent damage. Abscesses that remain untreated for prolonged periods of time can expand in size and even lead to intestinal obstruction or blood vessel invasion.<ref name = nitty /> | ||
<br><br> | <br><br> | ||
| Line 51: | Line 49: | ||
<i>B. fragilis</i> has inherent resistance to penicillin due to its production of β-lactamase.<ref name = nitty /> <i>Bacteroides</i> are also exposed to many antibiotics over the course of their lifetime as antibiotics first pass through the digestive tract. This constant exposure combined with its inherent resistance to penicillin leads to <i>Bacteroides</i> being one of the most antibiotic resistant microbes among anaerobic bacteria. | <i>B. fragilis</i> has inherent resistance to penicillin due to its production of β-lactamase.<ref name = nitty /> <i>Bacteroides</i> are also exposed to many antibiotics over the course of their lifetime as antibiotics first pass through the digestive tract. This constant exposure combined with its inherent resistance to penicillin leads to <i>Bacteroides</i> being one of the most antibiotic resistant microbes among anaerobic bacteria. | ||
Antibiotics such as clinimycin, metronidazole, carbapenem, beta-lactam/beta-lactamase inhibitor combinations (e.g. ampicillin/sulbactam, piperacillin/tazobactam), and cephamycins (cefoxitin, cefotetan, cefmetazole), are some of the only treatments found to eliminate a <i>Bacteroides fragilis</i> infection.<ref>Mandell GL, Bennett JE, Dolin R (2004). <i>Principles and Practice of Infectious Diseases</i> (6th ed.). Churchill Livingstone.</ref><ref>Brook I (December 2007). <i>Treatment of anaerobic infection</i>. Expert Rev Anti Infect Ther. 5 (6): 991–1006. </ref> However, resistance rates to cefoxitin and clindamycin are on the rise.<ref>Bartlett JG, Fabre V. <i>Bacteroides Fragilis</i>. In: Johns Hopkins ABX Guide. Johns Hopkins University; 2019.</ref> After severe infections, the | Antibiotics such as clinimycin, metronidazole, carbapenem, beta-lactam/beta-lactamase inhibitor combinations (e.g. ampicillin/sulbactam, piperacillin/tazobactam), and cephamycins (cefoxitin, cefotetan, cefmetazole), are some of the only treatments found to eliminate a <i>Bacteroides fragilis</i> infection.<ref>Mandell GL, Bennett JE, Dolin R (2004). <i>Principles and Practice of Infectious Diseases</i> (6th ed.). Churchill Livingstone.</ref><ref>Brook I (December 2007). <i>Treatment of anaerobic infection</i>. Expert Rev Anti Infect Ther. 5 (6): 991–1006. </ref> However, resistance rates to cefoxitin and clindamycin are on the rise.<ref>Bartlett JG, Fabre V. <i>Bacteroides Fragilis</i>. In: Johns Hopkins ABX Guide. Johns Hopkins University; 2019.</ref> After severe infections, the abscesses commonly need to be drained and the patient should continue treatment with antibiotics. Abscesses in the lungs can usually heal without intravenous treatment. | ||
Preventative measures of <i>B. fragilis</i> infections includes administration of antibiotics prior to abdominal or pelvic surgery.<ref name = nitty /> | Preventative measures of <i>B. fragilis</i> infections includes administration of antibiotics prior to abdominal or pelvic surgery.<ref name = nitty /> | ||
| Line 60: | Line 58: | ||
==Research== | ==Research== | ||
Research into <i>B. fragilis</i> is especially relevant today as it continues to increasingly become resistant to more and more antibiotics. A large majority of the <i>B. fragilis</i> research is currently being conducted on the the polysaccharide capsule and how this structure contributes to the virulence and | Research into <i>B. fragilis</i> is especially relevant today as it continues to increasingly become resistant to more and more antibiotics. A large majority of the <i>B. fragilis</i> research is currently being conducted on the the polysaccharide capsule and how this structure contributes to the virulence and abscess formation within the host.<ref>Tzianabos AO, Kasper DL, Onderdonk AB. Structure and function of Bacteroides fragilis capsular polysaccharides: relationship to induction and prevention of abscesses. Clin Infect Dis. 1995 Jun;20 Suppl 2:S132-40.</ref> | ||
<i>Bacteroides</i> are common alternatives in fecal indicator organism as they make up a large amount of the normal fecal bacterial flora. <i>Bacteroides</i> have a high degree of host specificity and typically have a low growth potential making them optimal indicators. Due to their sheer numbers, there is a greater likelihood of isolating <i>Bacteroides</i> over other traditional fecal indicators, such as <i>E. coli</i>. As fecal indicator organisms, <i>B. fragilis</i>, especially, can be useful for identifying situations of poor sanitation and risk of potential infections from pathogenic microorganisms.<ref>Fiksdal L, Maki JS, LaCroix SJ, Staley JT. Survival and detection of Bacteroides spp., prospective indicator bacteria. Appl Environ Microbiol. 1985 Jan;49(1):148-50.</ref> The use of <i>Bacteroides</i> as a fecal indicator can determine the potential presence of dangerous bacteria following a flood or sewage contamination in a building as well as identifying fecal contamination in recreational waters. Testing for <i>Bacteroides</i> is often done using PCR analysis. | <i>Bacteroides</i> are common alternatives in fecal indicator organism as they make up a large amount of the normal fecal bacterial flora. <i>Bacteroides</i> have a high degree of host specificity and typically have a low growth potential making them optimal indicators. Due to their sheer numbers, there is a greater likelihood of isolating <i>Bacteroides</i> over other traditional fecal indicators, such as <i>E. coli</i>. As fecal indicator organisms, <i>B. fragilis</i>, especially, can be useful for identifying situations of poor sanitation and risk of potential infections from pathogenic microorganisms.<ref>Fiksdal L, Maki JS, LaCroix SJ, Staley JT. Survival and detection of Bacteroides spp., prospective indicator bacteria. Appl Environ Microbiol. 1985 Jan;49(1):148-50.</ref> The use of <i>Bacteroides</i> as a fecal indicator can determine the potential presence of dangerous bacteria following a flood or sewage contamination in a building as well as identifying fecal contamination in recreational waters. Testing for <i>Bacteroides</i> is often done using PCR analysis. | ||
Latest revision as of 03:59, 12 December 2020
Classification
Higher Order Taxa: Kingdom: Bacteria, Phylum: Bacteroidetes, Class: Bacteroidia, Order: Bacteroidales, Family: Bacteroidaceae, Genus: Bacteroides
Species: Bacteroides fragilis (B. fragilis)
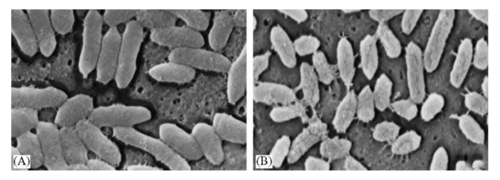
Introduction
Bacteroides fragilis is a gram-negative, non-spore forming, rod-like anaerobic microbe.[1] The bacteria reside in the gut flora of the human microbiota. Bacteroides fragilis remain commensal with the host when in the gut. There, they play an essential role in processing complex molecules into simpler ones in the host intestines. However, if the microbe enters the bloodstream or surrounding tissue it can lead to severe anaerobic infections, most commonly the formation of abscesses.
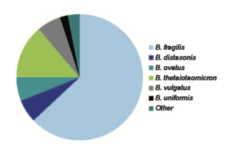
Anaerobes are the most common bacteria in the human colon. Within the group of anaerobes, Bacteroides make up approximately 25% of the anaerobic bacteria within the gut.[2] The Bacteroides genus consists of: B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B. vulgatus, B. uniformis, B. eggerthii, B. merdae, B. stercoris, and B. caccae (shown in Figure 2). Of these, B. fragilis is the most frequent isolate and responsible for the majority of anaerobic infections.[1] B. fragilis is most commonly associated with intra-abdominal infections.[3] A number of these species demonstrate resistance to a wide variety of current antibiotics.
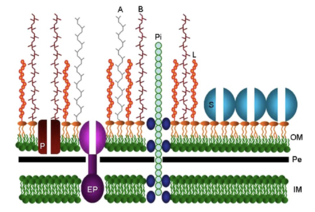
Cellular Structure
Elements of the cellular structure of B. fragilis contribute to its commensal relationship to the host or its advantages as a pathogen. Like all species of the Bacteroides genus, B. fragilis is a rod-like anaerobe with an inner cell membrane and an outer cell membrane containing lipopolysaccharides with specific side chains.[1] These side chains (polysaccharide A and polysaccharide B) are made up of repeating tetrasaccharide and hexasaccharide units, respectively(shown in Figure 3, labeled A and B). These polysaccharides consist of positively and negatively charged groups that interacted with each other to form a capsule that can be up to several hundred micrometers thick.[4] The polysaccharide complex is responsible for induction of abscess formation when the bacterium escapes the gut. There are also pili and efflux pumps protruding through the outer cell membrane(labeled EP and Pi). The pili are responsible for adherence to surrounding cells and mucus and the efflux pumps play a role in excreting toxins and antibiotics, contributing to the high level antibiotic resistance of B. fragilis.[5] B. fragilis also contain a large variation of proteins and enzymes that it uses to obtain and digest the wide variety of carbohydrates available in the gut. This is ideal for growth within the ever changing environment of the large intestine. This complex array of enzymes benefits the microbiome by breaking down nutrients for the other surrounding gut bacteria that are unable to digest the nutrients themselves. B. fragilis also produces a bile salt hydrolase to protect the anaerobe from the bile salts within in the gut. Without this enzyme, the bile salts would collapse its membrane and damage the cell.[4] B. fragilis also has the ability to adapt its surface architecture to the conditions within the gut or outside the gut. This function allows it to survive the changing and harsh conditions of the gut or evade the host immune system outside the gut. This complex cell surface structure allows for its dual existence as a commensal gut bacteria and, when escaped, a pathogen.
Pathogenesis and Epidemiology
B. fragilis only makes up about 1-2% of the gut micro-flora, yet it is the most predominant and persistent isolate found at the site of anaerobic infection.[4] Infections from B. fragilis commonly arise from a break in the mucosal membrane of the intestine.[6] The travel of B. fragilis outside of the gut can lead to a variety of infections such as local abscesses at the site of the mucosal disruption, metastatic abscesses by spread through the bloodstream to distant organs such as the abdomen, brain, pelvis, or liver, or abscesses formed in the lungs by aspiration of the microbe.[4]
Bacteroides species can be passed from a mother to offspring during childbirth, integrating itself into the human flora.[7] Species of Bacteroides, including B. fragilis, appear in the newborn microbiota about 10 days after birth.[8] Predisposition to B. fragilis infection can arise from surgery, disease, or trauma which can cause perforation of the gastrointestinal tract leading to pathogenesis.[1] The polysaccharide capsule created by the outer membrane of the cell contributes to the induction of abscess formation. When the host recognizes this polysaccharide complex, it triggers the formation of a fibrous wall that encapsulates the anaerobic colony.[4] Because the B. fragilis is anaerobic, it thrives without oxygen. Normally, an aerobic bacterial infection would be suffocated by the fibrous wall due to lack of oxygen; however, the anaerobic B. fragilis continues to grow and expand. The polysaccharide capsule combined with the anaerobic nature of the bacterium contributes to its powerful success as a pathogen.
E. coli, a facultative anaerobe, uses the oxygen at the site which brings the oxygen to a level where anaerobes, such as B. fragilis, can thrive and grow. Due to this phenomenon, many severe anaerobic infections contain a mix of anaerobic and aerobic flora.
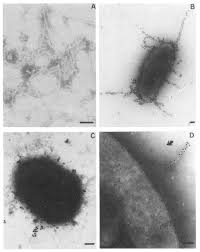
Virulence
Adherence to Tissues: The fimbriae(multiple pili) (shown in Figure 4 at left) of B. fragilis allows for easy adhesion to epithelial cells and extracellular molecules. B. fragilis can also adhere to peritoneal surfaces due to its unique polysaccharide capsules, a structural function that not many other anaerobes possess. These adhesive properties permit B. fragilis to establish itself within the host tissue and avoid immune targeting and attack.[1]
Protection from Host: The ability of a bacterium to protect itself from the immune response of its host is a large contributing factor to the species' virulence. Due to its polysaccharide capsule, a key promoter in the formation of intraabdominal abscesses, its LPS (Lipopolysaccharide), the main component of the outer membrane of gram-negative bacteria, and a variety of protective enzymes B. fragilis is able to defend itself from the immune response of the host.[1][9]
Destruction of Tissues: Histolytic enzymes of B. fragilis control and induce tissue destruction. The histolytic enzymes attack the extracellular matrix of the host, allowing for the infection to expand into this space.[10] This destruction of the host is one of the main reasons why B. fragilis is considered so virulent and dangerous. As the abscesses form, the B. fragilis also can eliminate surrounding tissue often leading to permanent damage. Abscesses that remain untreated for prolonged periods of time can expand in size and even lead to intestinal obstruction or blood vessel invasion.[1]
Treatment and Prevention
B. fragilis has inherent resistance to penicillin due to its production of β-lactamase.[1] Bacteroides are also exposed to many antibiotics over the course of their lifetime as antibiotics first pass through the digestive tract. This constant exposure combined with its inherent resistance to penicillin leads to Bacteroides being one of the most antibiotic resistant microbes among anaerobic bacteria.
Antibiotics such as clinimycin, metronidazole, carbapenem, beta-lactam/beta-lactamase inhibitor combinations (e.g. ampicillin/sulbactam, piperacillin/tazobactam), and cephamycins (cefoxitin, cefotetan, cefmetazole), are some of the only treatments found to eliminate a Bacteroides fragilis infection.[11][12] However, resistance rates to cefoxitin and clindamycin are on the rise.[13] After severe infections, the abscesses commonly need to be drained and the patient should continue treatment with antibiotics. Abscesses in the lungs can usually heal without intravenous treatment.
Preventative measures of B. fragilis infections includes administration of antibiotics prior to abdominal or pelvic surgery.[1]
Bacteroides infection can also be prevented by initial treatment with gentamicin, an antibiotic which targets facultative anaerobes such as E. coli but not Bacteroides. Because Bacteroides fragilis infection depends also on the existence of a facultative anaerobe, this antibiotic can be another preventative measure taken to prevent severe anaerobic infections involving B. fragilis. This method, however, does not stop the later development of Bacteroides abscesses. Therefore a more effective treatment combines both gentamicin with clindamycin, an antibiotic that is effective against Bacteroides, prevents the initial infection and later development of abdominal abscesses.[1]
Research
Research into B. fragilis is especially relevant today as it continues to increasingly become resistant to more and more antibiotics. A large majority of the B. fragilis research is currently being conducted on the the polysaccharide capsule and how this structure contributes to the virulence and abscess formation within the host.[14]
Bacteroides are common alternatives in fecal indicator organism as they make up a large amount of the normal fecal bacterial flora. Bacteroides have a high degree of host specificity and typically have a low growth potential making them optimal indicators. Due to their sheer numbers, there is a greater likelihood of isolating Bacteroides over other traditional fecal indicators, such as E. coli. As fecal indicator organisms, B. fragilis, especially, can be useful for identifying situations of poor sanitation and risk of potential infections from pathogenic microorganisms.[15] The use of Bacteroides as a fecal indicator can determine the potential presence of dangerous bacteria following a flood or sewage contamination in a building as well as identifying fecal contamination in recreational waters. Testing for Bacteroides is often done using PCR analysis.
Conclusion
B. fragilis is one of the most significant human anaerobic pathogens. It is the most commonly isolated bacteria from anaerobic infections but also exists commensally within the human intestines. The anaerobe only presents danger when it escapes the gastrointestinal tract due to a rupture. After escaping, the anaerobic infection can be very severe due to the high virulence and pathogenicity of B. fragilis. The pathogen can produce large abscesses outside the intestinal tract or even travel through the blood to infect a distant organ such as the abdomen, brain, liver, pelvis, or lungs. This specific species of Bacteroides is still being studied today due to its prominence within the body, its increasing antibiotic resistance, its important use in fecal tracing, and its unique structural and physical functions that other species of the Bacteroides genus do not contain. Continued understanding of the B. fragilis species can lead to a deeper understanding of the human microbiota and anaerobic infections.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007 Oct;20(4):593-621.
- ↑ Salyers, A. A. 1984. Bacteroides of the human lower intestinal tract. Annu. Rev. Microbial. 38:293-313.
- ↑ Goldstein, E. J. 1996. Anaerobic bactermia Clin. Infect. Dis. 23:97-101.
- ↑ 4.0 4.1 4.2 4.3 4.4 Pumbwe L, Skilbeck CA, Wexler HM. The Bacteroides fragilis cell envelope: quarterback, linebacker, coach-or all three? Anaerobe. 2006 Oct-Dec;12(5-6):211-20.
- ↑ Pumbwe L, Ueda O, Yoshimura F, Chang A, Smith R, Wexler HM. Bacteroides fragilis BmeABC efflux systems additively confer intrinsic antimicrobial resistance. J Antimicrob Chemother 2006; 58:37-46.
- ↑ Elsaghir H, Reddivari AKR. Bacteroides Fragilis. [Updated 2020 Jun 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-.
- ↑ Reid G. 2004. When microbe meets human. Clin. Infect. Dis.39:827-830.
- ↑ Simon, G. L., and S. L. Gorbach. 1984. Intestinal flora in health and disease. Gastroenterology 86:174-193.
- ↑ Mancuso, Giuseppe et al. Bacteroides fragilis-derived lipopolysaccharide produces cell activation and lethal toxicity via toll-like receptor 4. Infection and immunity vol. 73,9 (2005): 5620-7.
- ↑ Rudek, W. R. U. Haque. 1976. Extracellular enzymes of the genus Bacteroides. J. Clin. Microbiol. 4:458-460.
- ↑ Mandell GL, Bennett JE, Dolin R (2004). Principles and Practice of Infectious Diseases (6th ed.). Churchill Livingstone.
- ↑ Brook I (December 2007). Treatment of anaerobic infection. Expert Rev Anti Infect Ther. 5 (6): 991–1006.
- ↑ Bartlett JG, Fabre V. Bacteroides Fragilis. In: Johns Hopkins ABX Guide. Johns Hopkins University; 2019.
- ↑ Tzianabos AO, Kasper DL, Onderdonk AB. Structure and function of Bacteroides fragilis capsular polysaccharides: relationship to induction and prevention of abscesses. Clin Infect Dis. 1995 Jun;20 Suppl 2:S132-40.
- ↑ Fiksdal L, Maki JS, LaCroix SJ, Staley JT. Survival and detection of Bacteroides spp., prospective indicator bacteria. Appl Environ Microbiol. 1985 Jan;49(1):148-50.
Edited by Eva Illuzzi, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2020, Kenyon College.
