Lactobacillus acidophilus: Difference between revisions
No edit summary |
|||
| (49 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
{{Biorealm Genus}} | {{Biorealm Genus}} | ||
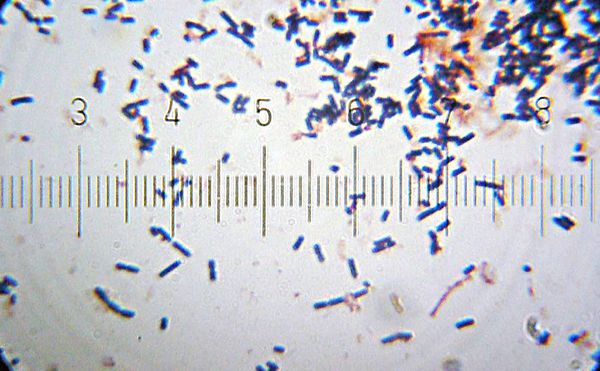
[[Image:20101212_200110_LactobacillusAcidophilus.jpg|thumb|600px|right|<i>Lactobacillus acidophilus</i><br/>Numbered ticks are 11 µM apart.<br/>[[Gram staining|Gram-stained]].<br/>Photograph by [[User:Blaylock|Bob Blaylock]].]] | |||
==Classification== | ==Classification== | ||
| Line 5: | Line 7: | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Bacteria; Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae | Bacteria; Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae | ||
===Species=== | ===Species=== | ||
'' | ''Lactobacillus acidophilus'' | ||
{| | {| | ||
| height="10" bgcolor="#FFDF95" | | | height="10" bgcolor="#FFDF95" | | ||
| Line 17: | Line 19: | ||
==Strains== | ==Strains== | ||
<b>Laboratory</b> | <b>Laboratory:</b> NCFM, 4962, CNRZ216, CNRZ218 | ||
<b>Human</b> | <b>Human:</b>HA1, HA2, HA3, HM2, HM6 | ||
<b>Pig</b> | <b>Pig:</b> PA3, PA12, PA19, P18, P47 | ||
<b>Chicken</b> | <b>Chicken:</b> C1, C2, C3, C7, C11 | ||
==Description and Significance== | ==Description and Significance== | ||
In general, ''Lactobacilli'' inhabit the gastrointestinal (GI) tract of humans and animals | In general, ''Lactobacilli'' is the largest genus of the lactic acid bacteria group and includes over 50 species. ''Lactobacilli'' commonly inhabit the gastrointestinal (GI) tract, oral, and vaginal regions of humans and animals. | ||
''Lactobacilli'' have many important roles in industry. They contribute to the production of some cheeses, yogurt, and other products. The lactic acid produced by ''Lactobacilli'' inhibits the growth of other organisms and lowers the pH of the product in these products. The starter cultures for such products are carefully cultivated and maintained because their metabolic end products contribute to the flavor of the final food product. Additionally, some of ''Lactobacilli's'' metabolic reactions are intentionally manipulated to breakdown milk proteins during cheese production. | |||
Further isolation and investigation into the physiological, biochemical, genetic, and fermentative properties have been widely explored in both humans and animals. The ''L. acidophilus'' strain, NCFM, has been commercially available in the United States as a probiotic strain since the mid-1970s. [http://www.pnas.org/cgi/content/full/102/11/3906 (2)] | Early studies of ''L. acidophilus'' were performed on strains isolated from fecal material of humans, pigs and chickens. Since then ''L. acidophilus'' has been further characterized as a short Gram-positive rod (2-10μm), is homofermentative and has optimal growth at temperatures of 37˚C-42˚C. Of the ''Lactobacillus'' species, ''L. acidophilus'' is the most well known and is commercially distributed as a probiotic. The World Health Organization defines a probiotic as "live microorganisms which, when administered in adequate amounts, confer a health benefit on the host". ([http://microbewiki.kenyon.edu/index.php/Lactobacillus_acidophilus#Ecology see Ecology]). [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=811162 (1)] | ||
Further isolation and investigation into the physiological, biochemical, genetic, and fermentative properties have been widely explored in both humans and animals. The ''L. acidophilus'' strain, NCFM, was isolated from a human in 1970 and characterized at North Carolina State University. NCFM has been commercially available in the United States as a probiotic strain since the mid-1970s. NCFM is also used for formula, yogurt and fluid milk production. [http://www.pnas.org/cgi/content/full/102/11/3906 (2)] | |||
==Genome structure== | ==Genome structure== | ||
''L. acidophilus'' NCFM contains no plasmids. [http://www.pnas.org/cgi/content/full/102/11/3906 (2)] | The complete circular genome of the NCFM strain of ''L. acidophilus'' contains 1,993,564 nucleotides. The DNA GC content was determined to be 34.71%. There are 1,864 open reading frames (ORFs) and 72.5% have been classified functionally. [http://www.pnas.org/cgi/content/full/102/11/3906 (2)] | ||
''L. acidophilus'' NCFM contains no plasmids. No complete prophages were found, but 9 phage-related integrases are predicted to remain as remnants. Additionally, 3 unique regions classified as potential autonomous units (PAUs) were identified. PAUs have unique characteristics that suggest that they are phage or plasmid remnants, but have not be further classified at this point. A unique signature sequence was noted. It is composed of 32 repeats of 29 base pairs (bps). [http://www.pnas.org/cgi/content/full/102/11/3906 (2)] | |||
''L. acidophilus'' NCFM produces a bacteriocin, called lactacin B that demonstrates antimicrobial activity against other ''Lactobacilli'' and ''Enterococcus faecalis''. The molecular mass of lactacin B is 6500 Daltons and is sensitive to proteinase K and pronase. It is stable between temperatures ranging from 121˚C to -20˚C. [http://jds.fass.org/cgi/reprint/84/2/319 (3)] | |||
Proteins that allow for ''L. acidophilus's'' unique metabolic functions and ability to survive acid conditions were also identified. These include gene clusters that allow for transport of a diverse group of carbohydrates, such as fructooligosaccharides and raffinose, and were often associated with transcriptional regulators. Coupled regulatory systems between acid tolerance and bacteriocin production are predicted in 9 locations. Overall these genes enable ''L. acidophilus'' to survive in the harsh GI conditions and promote interaction with the intestinal mucosa and microbiota. [http://jds.fass.org/cgi/reprint/84/2/319 (3)] | |||
==Cell structure and metabolism== | ==Cell structure and metabolism== | ||
''L. acidophilus'' grows in low pH (<3.5), anaerobic conditions and undergoes fermentation only. [http://www.blackwell-synergy.com/doi/full/10.1046/j.1365-2958.1999.01557.x (4)] | |||
In 1999, an H<sup>+</sup> induced ATPase was identified in ''L. acidophilus''. Based on primary structure and the genetic organization, it was further classified as a F<sub>1</sub>F<sub>0</sub>-type ATPase. Its similarity to the streptococcal ATPase and the H<sup>+</sup> inducibility of the operon suggests that it is responsible for an ATP-dependent exclusion of protons in order to maintain cytoplasmic pH (~7). [http://www.blackwell-synergy.com/doi/full/10.1046/j.1365-2958.1999.01557.x (4)] | |||
''L. acidophilus'' lack cytochromes, porphyrins, and respiratory enzymes and as a result are unable to undergo any oxidative phosphorylation or respiration. Because they utilize sugars as their substrates for fermentation, they inhabit environments with high sugar abundance, such as the GI tract in humans and animals. More specifially, ''L. acidophilus'' is homofermentative which means that the only byproduct it forms from fermentation is lactic acid. For every one glucose molecule that undergoes fermentation in ''L. acidophilus'', the energy yield is two ATPs. As a result, homofermentative microbes must catabolize large amounts of substrate to generate enough energy for growth. In addition to glucose, ''L. acidophilus'' utilizes aesculin, cellobiose, galactose, lactose, maltose, salicin, sucrose, and trehalose for fermentation. | |||
==Ecology== | |||
''L. acidophilus'' is best known as a probiotic. The exact mechanism of the probiotic effect is still under investigation. ([http://microbewiki.kenyon.edu/index.php/Lactobacillus_acidophilus#Current_Research see Current Research]) | |||
Adherence and colonization is one of many suggested mechanisms responsible for the probiotic effect of ''L. acidophilus''. Adherence and colonization of the intestinal epithelium can act in two ways: (1) competition for space on the epithelium and (2) interaction with enterocytes. There is some evidence that ''L. acidophilus'' NCFM has the ability to adhere through a protein mediated mechanism. ([http://microbewiki.kenyon.edu/index.php/Lactobacillus_acidophilus#Current_Research see Current Research]) After ''L. acidophilus'' has colonized the GI tract, as a probiotic bacteria it has the potential to influence the existing microbial population in favor of the host's health. [http://jds.fass.org/cgi/reprint/84/2/319 (3)] | |||
Antimicrobial activity is considered an important mechanism by which probiotic bacteria act to inhibit a range of microbes that have potentially detrimental effects. It is suggested that ''L. acidophilus'' produces bacteriocins (proteins that are active against other bacteria). This specific mechanism is currently being researched ([http://microbewiki.kenyon.edu/index.php/Lactobacillus_acidophilus#Current_Research see Current Research]). [http://jds.fass.org/cgi/reprint/84/2/319 (3)] | |||
''L. acidophilus'' has been suggested as a supplement for lactose intolerant individuals. When taken orally and in sufficient dosages, there is evidence for a decrease in symptoms of lactose maldigestion. Presumably, the ''L. acidophilus'' colonizes the GI tract and contributes to the metabolism of lactose during digestion and transit through the GI tract. [http://jds.fass.org/cgi/reprint/84/2/319 (3)] | |||
==Current Research== | |||
===Protein Mediated Adherence=== | |||
''L. acidophilus'' | Adherence to the epithelium of the GI tract is thought to play an important role in the probiotic effect of ''L. acidophilus''. Stuies show that the exact mechanism of adherence varies from strain to strain. Possible mechanisms include protein and carbohydrate mediated adherence. Although both types of mechanisms have been demonstrated ''in vitro'' neither has successfully been demonstrated ''in vivo''. Because the GI tract is a constantly changing environment it is difficult to mimic the environment ''in vitro''. Further studies with biopsies of intestinal tissues are necessary to confirm adherence and retention of ''L. acidophilus'' in the GI tract. However, studies that include such biopsies are rare. ''In vitro'', NCFM specifically shows a protein mediated response. The NCFM that demonstrated adherence did not appear to have a polysaccharide layer, which may be significant to its ability to adhere. Further study is needed to confirm mechanisms ''in vivo''. [http://jds.fass.org/cgi/reprint/84/2/319 (3)] | ||
== | ===Innate Antimicrobial Activity=== | ||
The | When ''L. acidophilus'' is co-cultivated with other organisms, ''L. acidophilus'' has repeatedly been shown to inhibit the growth of competing microbes. It is thought that ''L. acidophilus'' produces a variety of antimicrobial compounds including organic acids, hydrogen peroxide, diacetyl and bacteriocins. The activity of these compounds is evident in the laboratory, but the ''in vivo'' role of these compounds is less clear. This is an area of active research. For instance, human fecal samples show a correlation between a reduction in pH and an increase in short chain fatty acids with higher fecal counts of ''Lactobacilli'' and ''bifidobacteria'' (which is another species that exhibits a probiotic effect). In the laboratory strain NCFM demonstrated antagonistic activity against common foodborne disease agents such as ''Staphylcoccus aureus'', ''Salmonella typhimurium'', and enteropathogenis ''Escherichia coli''. [http://jds.fass.org/cgi/reprint/84/2/319 (3)] | ||
===Probiotic Effects Beyond the GI Tract=== | |||
''L. acidophilus'' has long been considered a probiotic conferring digestive benefits to its host. Now research is beginning to examine ''L. acidophilus''' potential probiotic effect in combating vulvovaginal candidiasis (VVC). VVC is associated with a low number of ''Lactobacilli'' in the vagina or with peroxide-non-producing vaginal ''Lactobacilli''. A review of the literature shows no clear indication of whether or not ''L. acidophilus'' is effective in all VVC cases. The studies are preliminary and had methodological problems such as small sample size, lacked a control group and included women without confirmed VVC. Further studies are necessary with appropriate experimental design. Additionally, studies regarding oral or intravaginal dosage are necessary to determine a more efficient administration of the probiotics. [http://jac.oxfordjournals.org/cgi/content/full/58/2/266 (5)] | |||
After section ‘Probiotic Effects Beyond the GI Tract’ I would like to add a section named ‘Lactobacillus in Yogurt and vaginal flora”: | |||
Most common bacteria found in a healthy vagina are lactobacillus species, the same bacteria commonly found in yogurt. In case you were wondering, Cecilia Westbrook, an MD/PhD student at the University of Wisconsin, Madison was able to successfully make yogurt from lactobacillus that she cultured from her vagina. However, it is not recommended that we produce yogurt from microbes found from the human body because of potential pathogenic consequences if inappropriately isolated. | |||
"Yogurt-Lactobacillus is not all that different from the vaginal Lactobacilli," explained Hertzberger. "They all produce heaps of lactic acid, both in the vagina and in yogurt." (news.health.com) | |||
==References== | ==References== | ||
| Line 65: | Line 90: | ||
2. [http://www.pnas.org/cgi/content/full/102/11/3906 Altermann, E., Russell, W.M., Azcarate-Peril, M.A., et al. "Complete genome sequence of the probiotic lactic acid bacterium ''Lactobacillus acidophilus'' NCFM". ''Proceedings of the National Academy of Sciences of the United States''. 2005. Volume 102. No. 11.] | 2. [http://www.pnas.org/cgi/content/full/102/11/3906 Altermann, E., Russell, W.M., Azcarate-Peril, M.A., et al. "Complete genome sequence of the probiotic lactic acid bacterium ''Lactobacillus acidophilus'' NCFM". ''Proceedings of the National Academy of Sciences of the United States''. 2005. Volume 102. No. 11.] | ||
3. [http://www.blackwell-synergy.com/doi/full/10.1046/j.1365-2958.1999.01557.x Kullen, MJ and Klaenhammer, T.R. "Identification of the pH-inducible, proton-translocating F<sub>1</sub>F<sub>0</sub>ATPase (atpBEFHAGDC) operon of ''Lactobacillus acidophilus'' by differential | 3. [http://jds.fass.org/cgi/reprint/84/2/319 Sanders, M.E. and Klaenhammer, T.R. "The Scientific Basis of ''Lactobacillus acidophilus'' NCFM Functionally as a Probiotic". ''Journal of Dairy Sciences''. 2001. Volume 84. Pages 319-331.] | ||
4. [http://www.blackwell-synergy.com/doi/full/10.1046/j.1365-2958.1999.01557.x Kullen, MJ and Klaenhammer, T.R. "Identification of the pH-inducible, proton-translocating F<sub>1</sub>F<sub>0</sub>ATPase (atpBEFHAGDC) operon of ''Lactobacillus acidophilus'' by differential display; gene structure, cloning and characterization". ''Molecular Biology''. 1999. Volume 33. Pages 1152-1161.] | |||
5. [http://jac.oxfordjournals.org/cgi/content/full/58/2/266 Falagas, M.E., Betsi, G.I. and Athanasiou, S. "Probiotics for prevention of recurrent vulvovaginal candidiasis: a review". ''Journal of Antimicrobial Chemotherapy''. 2006. Volume 58. Pages 266-272.] | |||
Edited by [mailto:jennbs4@yahoo.com Jennifer B. Samore], student of [mailto:ralarsen@ucsd.edu Rachel Larsen] and Kit Pogliano | Edited by [mailto:jennbs4@yahoo.com Jennifer B. Samore], student of [mailto:ralarsen@ucsd.edu Rachel Larsen] and Kit Pogliano | ||
6. http://www.sciencealert.com/a-researcher-is-making-yoghurt-from-her-private-bacteria-because-science | |||
7. http://news.health.com/2015/02/18/why-this-woman-made-yogurt-with-her-vaginal-secretions/ | |||
Latest revision as of 22:16, 12 May 2016
A Microbial Biorealm page on the genus Lactobacillus acidophilus
Classification
Higher order taxa
Bacteria; Firmicutes; Bacilli; Lactobacillales; Lactobacillaceae
Species
Lactobacillus acidophilus
Strains
Laboratory: NCFM, 4962, CNRZ216, CNRZ218
Human:HA1, HA2, HA3, HM2, HM6
Pig: PA3, PA12, PA19, P18, P47
Chicken: C1, C2, C3, C7, C11
Description and Significance
In general, Lactobacilli is the largest genus of the lactic acid bacteria group and includes over 50 species. Lactobacilli commonly inhabit the gastrointestinal (GI) tract, oral, and vaginal regions of humans and animals.
Lactobacilli have many important roles in industry. They contribute to the production of some cheeses, yogurt, and other products. The lactic acid produced by Lactobacilli inhibits the growth of other organisms and lowers the pH of the product in these products. The starter cultures for such products are carefully cultivated and maintained because their metabolic end products contribute to the flavor of the final food product. Additionally, some of Lactobacilli's metabolic reactions are intentionally manipulated to breakdown milk proteins during cheese production.
Early studies of L. acidophilus were performed on strains isolated from fecal material of humans, pigs and chickens. Since then L. acidophilus has been further characterized as a short Gram-positive rod (2-10μm), is homofermentative and has optimal growth at temperatures of 37˚C-42˚C. Of the Lactobacillus species, L. acidophilus is the most well known and is commercially distributed as a probiotic. The World Health Organization defines a probiotic as "live microorganisms which, when administered in adequate amounts, confer a health benefit on the host". (see Ecology). (1)
Further isolation and investigation into the physiological, biochemical, genetic, and fermentative properties have been widely explored in both humans and animals. The L. acidophilus strain, NCFM, was isolated from a human in 1970 and characterized at North Carolina State University. NCFM has been commercially available in the United States as a probiotic strain since the mid-1970s. NCFM is also used for formula, yogurt and fluid milk production. (2)
Genome structure
The complete circular genome of the NCFM strain of L. acidophilus contains 1,993,564 nucleotides. The DNA GC content was determined to be 34.71%. There are 1,864 open reading frames (ORFs) and 72.5% have been classified functionally. (2)
L. acidophilus NCFM contains no plasmids. No complete prophages were found, but 9 phage-related integrases are predicted to remain as remnants. Additionally, 3 unique regions classified as potential autonomous units (PAUs) were identified. PAUs have unique characteristics that suggest that they are phage or plasmid remnants, but have not be further classified at this point. A unique signature sequence was noted. It is composed of 32 repeats of 29 base pairs (bps). (2)
L. acidophilus NCFM produces a bacteriocin, called lactacin B that demonstrates antimicrobial activity against other Lactobacilli and Enterococcus faecalis. The molecular mass of lactacin B is 6500 Daltons and is sensitive to proteinase K and pronase. It is stable between temperatures ranging from 121˚C to -20˚C. (3)
Proteins that allow for L. acidophilus's unique metabolic functions and ability to survive acid conditions were also identified. These include gene clusters that allow for transport of a diverse group of carbohydrates, such as fructooligosaccharides and raffinose, and were often associated with transcriptional regulators. Coupled regulatory systems between acid tolerance and bacteriocin production are predicted in 9 locations. Overall these genes enable L. acidophilus to survive in the harsh GI conditions and promote interaction with the intestinal mucosa and microbiota. (3)
Cell structure and metabolism
L. acidophilus grows in low pH (<3.5), anaerobic conditions and undergoes fermentation only. (4)
In 1999, an H+ induced ATPase was identified in L. acidophilus. Based on primary structure and the genetic organization, it was further classified as a F1F0-type ATPase. Its similarity to the streptococcal ATPase and the H+ inducibility of the operon suggests that it is responsible for an ATP-dependent exclusion of protons in order to maintain cytoplasmic pH (~7). (4)
L. acidophilus lack cytochromes, porphyrins, and respiratory enzymes and as a result are unable to undergo any oxidative phosphorylation or respiration. Because they utilize sugars as their substrates for fermentation, they inhabit environments with high sugar abundance, such as the GI tract in humans and animals. More specifially, L. acidophilus is homofermentative which means that the only byproduct it forms from fermentation is lactic acid. For every one glucose molecule that undergoes fermentation in L. acidophilus, the energy yield is two ATPs. As a result, homofermentative microbes must catabolize large amounts of substrate to generate enough energy for growth. In addition to glucose, L. acidophilus utilizes aesculin, cellobiose, galactose, lactose, maltose, salicin, sucrose, and trehalose for fermentation.
Ecology
L. acidophilus is best known as a probiotic. The exact mechanism of the probiotic effect is still under investigation. (see Current Research)
Adherence and colonization is one of many suggested mechanisms responsible for the probiotic effect of L. acidophilus. Adherence and colonization of the intestinal epithelium can act in two ways: (1) competition for space on the epithelium and (2) interaction with enterocytes. There is some evidence that L. acidophilus NCFM has the ability to adhere through a protein mediated mechanism. (see Current Research) After L. acidophilus has colonized the GI tract, as a probiotic bacteria it has the potential to influence the existing microbial population in favor of the host's health. (3)
Antimicrobial activity is considered an important mechanism by which probiotic bacteria act to inhibit a range of microbes that have potentially detrimental effects. It is suggested that L. acidophilus produces bacteriocins (proteins that are active against other bacteria). This specific mechanism is currently being researched (see Current Research). (3)
L. acidophilus has been suggested as a supplement for lactose intolerant individuals. When taken orally and in sufficient dosages, there is evidence for a decrease in symptoms of lactose maldigestion. Presumably, the L. acidophilus colonizes the GI tract and contributes to the metabolism of lactose during digestion and transit through the GI tract. (3)
Current Research
Protein Mediated Adherence
Adherence to the epithelium of the GI tract is thought to play an important role in the probiotic effect of L. acidophilus. Stuies show that the exact mechanism of adherence varies from strain to strain. Possible mechanisms include protein and carbohydrate mediated adherence. Although both types of mechanisms have been demonstrated in vitro neither has successfully been demonstrated in vivo. Because the GI tract is a constantly changing environment it is difficult to mimic the environment in vitro. Further studies with biopsies of intestinal tissues are necessary to confirm adherence and retention of L. acidophilus in the GI tract. However, studies that include such biopsies are rare. In vitro, NCFM specifically shows a protein mediated response. The NCFM that demonstrated adherence did not appear to have a polysaccharide layer, which may be significant to its ability to adhere. Further study is needed to confirm mechanisms in vivo. (3)
Innate Antimicrobial Activity
When L. acidophilus is co-cultivated with other organisms, L. acidophilus has repeatedly been shown to inhibit the growth of competing microbes. It is thought that L. acidophilus produces a variety of antimicrobial compounds including organic acids, hydrogen peroxide, diacetyl and bacteriocins. The activity of these compounds is evident in the laboratory, but the in vivo role of these compounds is less clear. This is an area of active research. For instance, human fecal samples show a correlation between a reduction in pH and an increase in short chain fatty acids with higher fecal counts of Lactobacilli and bifidobacteria (which is another species that exhibits a probiotic effect). In the laboratory strain NCFM demonstrated antagonistic activity against common foodborne disease agents such as Staphylcoccus aureus, Salmonella typhimurium, and enteropathogenis Escherichia coli. (3)
Probiotic Effects Beyond the GI Tract
L. acidophilus has long been considered a probiotic conferring digestive benefits to its host. Now research is beginning to examine L. acidophilus' potential probiotic effect in combating vulvovaginal candidiasis (VVC). VVC is associated with a low number of Lactobacilli in the vagina or with peroxide-non-producing vaginal Lactobacilli. A review of the literature shows no clear indication of whether or not L. acidophilus is effective in all VVC cases. The studies are preliminary and had methodological problems such as small sample size, lacked a control group and included women without confirmed VVC. Further studies are necessary with appropriate experimental design. Additionally, studies regarding oral or intravaginal dosage are necessary to determine a more efficient administration of the probiotics. (5)
After section ‘Probiotic Effects Beyond the GI Tract’ I would like to add a section named ‘Lactobacillus in Yogurt and vaginal flora”:
Most common bacteria found in a healthy vagina are lactobacillus species, the same bacteria commonly found in yogurt. In case you were wondering, Cecilia Westbrook, an MD/PhD student at the University of Wisconsin, Madison was able to successfully make yogurt from lactobacillus that she cultured from her vagina. However, it is not recommended that we produce yogurt from microbes found from the human body because of potential pathogenic consequences if inappropriately isolated.
"Yogurt-Lactobacillus is not all that different from the vaginal Lactobacilli," explained Hertzberger. "They all produce heaps of lactic acid, both in the vagina and in yogurt." (news.health.com)
References
Edited by Jennifer B. Samore, student of Rachel Larsen and Kit Pogliano
6. http://www.sciencealert.com/a-researcher-is-making-yoghurt-from-her-private-bacteria-because-science
7. http://news.health.com/2015/02/18/why-this-woman-made-yogurt-with-her-vaginal-secretions/