Spoiled meat niche: Difference between revisions
No edit summary |
|||
| (183 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | |||
[[Image:beef.jpg|left|500x500px|frame|beef]] | |||
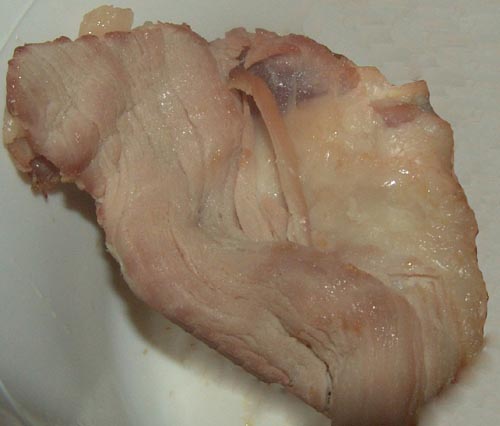
[[Image:Spoiled meat.jpg|right|250x250px|frame|semi-cooked pork defrosted, placed in container at room temperature: after Day 1, odor can be observed [30]]] | |||
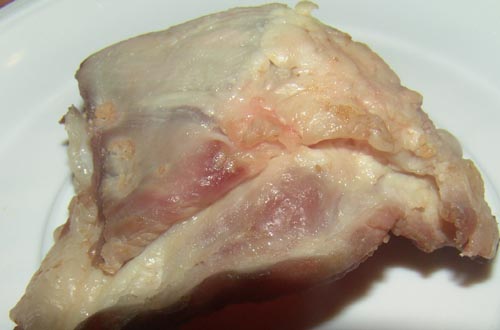
[[Image:Spoiled meat day 2.jpg|250x250px|frame|semi-cooked pork defrosted, placed in container at room temperature: Day 2 [30]]] | |||
==Introduction to Spoiled Meat== | |||
Meat is a complex niche that has chemical and physical properties which allow the colonization and development of a variety of microorganisms. Many factors can influence which microbes are present on certain meat. After slaughtering, meat can be contaminated with bacteria from the water, air, and soil as well as from the workers and the equipments involved during the manufacturing process. The point of spoilage is normally defined as the maximum acceptable bacterial level, the unacceptable odor/flavor, or the appearance for consumption. [2] However, the common features of spoilage consist of discoloration, gas production, and foul odor. Most of the organisms that live within this niche lives in an atmosphere of a pH under 7 and around 4-7°C. The living conditions might change which will determine the rate of organism production. Although there are many different types of meat, most of the organisms that grow in this niche are similar. This page will focus on the three most common sources of meat: pork, chicken, and beef. Spoiled meat varies in different forms, from being raw, marinated, or being cooked and cured. The spoiled meat is stored in a vacuum or modified atmosphere.[16] | |||
==Description of Niche== | ==Description of Niche== | ||
===Physical Conditions | ===Physical Conditions=== | ||
Because of its significant nutrients available on the surface and its high water content, meat is one of the most perishable foods. The conditions of spoiled meat vary on the type of meat, how the meat was prepared, and how the meat is stored. | Because of its significant nutrients available on the surface and its high water content, meat is one of the most perishable foods. The conditions of spoiled meat vary on the type of meat, how the meat was prepared, and how the meat is stored. | ||
Pork | ====Pork==== | ||
The follow conditions are for Frankfurter-type sausages and sliced pork. For meat that is initially cooked/heated at 65-75º C [ | The follow conditions are for Frankfurter-type sausages and sliced pork. For meat that is initially cooked/heated at 65-75º C [26] and stored in a vacuum or modified atmosphere, at a temperature of 4º C, the salt content is around 2%, with a pH of above 6, making it a generally neutral environment. The moisture is 61.5% in Frankfurter-type sausages and 68% in sliced pork [16]. | ||
Chicken | ====Chicken==== | ||
Marinated chicken meat in a modified atmosphere (MA) packaged and stored at temperatures ranging from 3.4 to 7.7ºC. The marinating factor includes many sub-ingredients such as acidic sauce including sucrose or glucose, salt, and spices that adds to the flavor of meat. [ | Marinated chicken meat in a modified atmosphere (MA) packaged and stored at temperatures ranging from 3.4 to 7.7ºC. The marinating factor includes many sub-ingredients such as acidic sauce including sucrose or glucose, salt, and spices that adds to the flavor of meat. [13] The marinate provides an acidic pH of 4.7 to 5.0. [13] | ||
Beef | ====Beef==== | ||
For ground beef (fresh from the market) stored aerobically (high oxygen MA) at the temperature of 5-7ºC, chilled- refrigerated, the pH was 5.5 to 5.92. [ | For ground beef (fresh from the market) stored aerobically (high oxygen MA) at the temperature of 5-7ºC, chilled- refrigerated, the pH was 5.5 to 5.92. [12] For raw beef steaks, the pH was 5.4 to 5.6 the lactic acid bacteria level was 2.2 × 104 CFU/g [28]. The beef steak meat packages were also moisture enhanced with levels between 112 and 115% of original weight [28]. | ||
===Influence by Adjacent Communities | ===Influence by Adjacent Communities=== | ||
Because it is stored in a modified, vacuum, refrigerated atmosphere, the spoiled meat niche is not close to another niche, but the handling and process of packaging meat and can often add microflora to the products. An estimated .5-2 log | Because it is stored in a modified, vacuum, refrigerated atmosphere, the spoiled meat niche is not close to another niche, but the handling and process of packaging meat and can often add microflora to the products. An estimated .5-2 log CFU/g of bacteria is added to the meat due to these processes. [26] | ||
===Conditions under which the environment changes=== | ===Conditions under which the environment changes=== | ||
The influenced on spoiled meat is by the microflora on the meat itself, mainly <i>Lactobacillus sp.</i> and <i>Leuconostoc sp.</i>, and human added Lactic acid bacteria. Lactic acid bacteria are antagonistic cultures added to the meat in order to inhibit pathogens and increase the shelf life, which are protective cultures as a means of biopreservation. In this way, Lactic acid changes the condition of the niche by inhibiting unwanted microorganisms, via several methods, such as the production of bacteriocins and enzymes, and also by simply competing for nutrients in the same niche. | The influenced on spoiled meat is by the microflora on the meat itself, mainly <i>Lactobacillus sp.</i> and <i>Leuconostoc sp.</i>, and human added Lactic acid bacteria. Lactic acid bacteria are antagonistic cultures added to the meat in order to inhibit pathogens and increase the shelf life, which are protective cultures as a means of biopreservation. In this way, Lactic acid changes the condition of the niche by inhibiting unwanted microorganisms, via several methods, such as the production of bacteriocins and enzymes, and also by simply competing for nutrients in the same niche. | ||
The niche community changes as the shelf life continues. | The niche community changes as the shelf life continues. If stored properly, either refrigerated or in an anaerobic atmosphere, lactic acid bacteria can prevent microflora from spoiling the meat too quickly, and this will allow other microbes to participate in the spoilage process. [26] As the shelf life reaches its limit, Lactic acid bacteria along with other bacterial populations increase which causes spoilage. Depending on the product handling after cooking, the spoiled pork may be recontaminated with up to 0.5-2 log CFU/g of total bacteria. [26] | ||
In frankfurter-type sausages, the presence of <i>Lact. curvatus</i> can potentially change the physical conditions of the niche by decreasing the pH of the spoiled meat (from 6.6 to 5.8) at the end of its shelf life, after 28 days. In pork, the presence of Lactic Acid bacteria caused a decrease of pH from 6.6 to 5.1-5.3 (vacuum) and 5.5-5.6 (modified atmosphere) at the end of its shelf life. [16] | |||
The conditions of the niche consists can cause various changes ranging from souring, changing the flavor, texture, and color, producing gas and slime, and change in the pH level. Spoilage affects can be noticed at various points in the shelf life, as early as a few days and up to the end of the shelf life (18 to 42 days), where the meat is dominated by spoilage. [4] Meat often shows a distinct color change from a reddish meat color to a brown pigment and shows bulging due to gas formation after packaging. [8] Spoilage is also apparent by swelling of packages and characterized by a green coloring due to production of hydrogen peroxide [28] with a strong buttery odor due to putrescine and cadaverine. [13] In addition, samples of meat loses water during storage (weight loss). Slime formation may occur, such as biofilms at low temperatures and have quorum sensing which also produces pigment changes that may not be suitable for human consumption. The development of spoilage is also associated to microbial consumption of meat nutrients such as sugars and amino acids. [8] | |||
====<b>The influence of Chemicals</b><br>==== | |||
Increasing concentrations of CO<sub>2</sub>, a decreased pH, and a chilled storage are used to preserve meat and prevent spoilage microflora from forming [12]. But still, the dominant organisms <i>Lactobacillus oligofermentans</i> and <i>[[Pseudomonas]]</i> are present. | |||
Marinating chicken often maintains the low pH because of the low pH of the marinate. The process of marinating in chicken can support the growth of <i>Lactobacillus oligofermentans</i> in which its population can grow up to 10<sup>8</sup> to 10<sup>10</sup> CFU/g. [13] Ingredients also used to promote flavoring and taste of beef/steak and vacuum packaging with glucose and sugar can expedite the spoilage process that is depicted through a green coloring and a strong buttery odor of the meat. [28] | |||
In the experiment Spoilage of value-added, high-oxygen modified-atmosphere packaged raw beef steaks by <i>[[Leuconostoc]] gasicomitatum</i> and <i>Leuconostoc gelidum</i>, the effects of O<sub>2</sub> and CO<sub>2</sub> content was studied via three packaging conditions of beef. All conditions were modified atmosphere packaged but with different concentrations of O<sub>2</sub> and CO<sub>2</sub>: MAP1 consists of only air; MAP2 is 60% O<sub>2</sub> and 40% CO<sub>2</sub>; and MAP3 is 20% O<sub>2</sub> and 40% CO<sub>2</sub>. [8] These three varying packaging conditions show different effects on the spoiling piece of meat. The conditions for MAP2 protect the spoilage of meat (little color change of the beef and little microbial loads) after refrigerated storage compared to MAP1 and MAP3. According to the findings of different packaging condition studies, many different changes occurred. For MAP1 where only oxygen is present, the concentration of O<sub>2</sub> decreased from 21% to 0% and concentrations of CO<sub>2</sub> increased from 0% to 25% in 14 days.[8] In the MAP2 condition, both concentrations of O<sub>2</sub> and CO<sub>2</sub> remain constant. [8] For MAP3, the same observations is seen for the first seven days, but during the next seven days (day 7-14), a drop in oxygen concentration and an increase in CO<sub>2</sub> concentration is detected. [8] | |||
==Who lives there?== | |||
Meat provides a suitable niche for many microorganisms because it has a rich source of nutrients.The main microbes that are responsible for meat spoilage consist of <i>Brochotrix thermosphacta</I>, <I>Carnobacterium</I>, <I>[[Clostridium]]</I>, <I>Enterobacterium</I>, <I>[[Leuconostoc]]</I>, and <I>[[Pseudomonas]]</I>. | |||
===Microbes Present=== | |||
====<b><I>Brochothrix thermosphacta</I></b><br>==== | |||
<i>Brochothrix Thermosphacta</I> is a microorganism for which meat is considered an ecological niche. Its ability to grow under both aerobic and anaerobic conditions makes a significant meat colonizer. The genus <i>Brochothrix</i> is characterizes as gram-positive, non-spore forming, non-motile, catalase-positive, facultatively anaerobic, regular rod shaped bacteria. The optimal temperature for growth is 20-25º C. The optimal pH for <i>B. thermosphacta</i> to grow is pH 7.0 but growth is seen within the ranges of pH 5-9. <i>Brochothrix thermosphacta</i> is more resistant to irradiation than common meat spoilage organism such as <i>[[Pseudomonas]]</I> but are affected by irradiation does of 0.5 to 2.0 kilogray. The species have often been isolated from irradiated meat and poultry. Although, they are an important spoilage organism found prepacked meats and in meat stored in chill temperature, they can also inhabit other niches such frozen foods, milk, and cream. Storage conditions often selectively favor its growth. <i>Brochothrix ssp </I> can grow at temperatures a low as 0ºC and under conditions of low oxygen concentration and high C0<sub>2</sub> concentration. For metabolism, <i>Brochothrix thermosphacta</I> has enzymes for both the hexose-monophosphate and glycolysis pathways of glucose. Fermentative metabolism of glucose always results in the production of L+ lactic acid, but other end products depend on growth conditions [24]. Major end products of aerobic metabolism of glucose by <i>B. Thermosphacta</i> growing on meat are acetoin and acetic, isobutyric, isovaleric and 2 methylbutyric acid. In minimal medium, glucose is the source of all the end products; However, in complex medium such as meat, only acetoin and acetic acid are derived from glucose; Isobutyric, isovaleric, and 2-methylbutyric acids are produced from valine, leucine, and isoleucine, respectively[5]. These compounds, or their derivatives, are responsible for the odor that often characterizes spoiled meat. Unlike proteolytic spoilage bacteria such as <i>[[Pseudomonas]]</I>, <i>B. thermosphacta</I> is usually found only on the meat surface. In prepacked meat, it grows in the area between the meat-plastic film [24]. | |||
<br> | |||
====<b><I>Carnobacterium</I></b><br>==== | |||
<I>Carnobacterium (C.)</I> is gram-positive genus and contains nine species: <I>C. divergens</I>, <I>C. maltaromaticum</I>, <I>C altherfunditum</I>, <I>C. funditum</I>, <I>C. gallinarum</I>, <I>C. mobile</I>, <I>C. inhibens</I>, <I>C. pleistocenium</I>, and <I>C. viridans</I>. However, only <I>C. divergens</I> and <I>C. maltaromaticium</I> can frequently be isolated in meat products. In food, they are often found responsible for producing antimicrobial peptides and bacteriocin. <I>C. divergens</I> and <I>C. maltaromaticum</I> grow anaerobically at high CO2 concentration, low concentration and high pressure. They are able to metabolize arginine and other carbohydrates, which may contribute to their adjustment to the environment. Their catabolic activities were observed to play a role in sensory spoilage of meat products. The other seven species are not commonly encountered. One example of its effects on food is that, <I>C. divergens</I> can produce H<sub>2</sub>O<sub>2</sub>, and when it encounters <I>C. vriridans</I>, it would result in a green discoloration of ham. However, this genus has not been fully understood. Its full genome sequence has yet been determined. Thus more studies are needed for a full understanding of <I>Carnobacterium</I> effects in food. [15] | |||
<br> | |||
====<b><I>[[Clostridium]]</I></b><br>==== | |||
[[Image:ClostridiumTetani.jpg|right|100x200px|frame|Clostridium tetani]] | |||
<I>Clostridium</I> is a rod-shaped cell with a gram-positive membrane. These microbes are anaerobes and some are toxin-producing pathogens. These pathogens include <I>[[Clostridium difficile]]</I>, <I>[[Clostridium botulinum]]</I>, <I>[[Clostridium tetani]]</I>, and <I>[[Clostridium perfringens]]</I>. Some of them produce acetone, butanol, ethanol, isopropanol, and organic acids. This bacterium can go through spore formation for survival. <I>[[Clostridium]]</I> produces large amounts of gas in packaged meat. It is usually coupled up with foul odors and causes the package to appear in a blown pack. [20] Aside from finding this bacterium in spoiled meat, it can also be found in soil, sewage, and animal intestines. The toxin produced by this bacterium can do harm and help heal. So far this toxin has helped treat dystonias (neurological diseases involved abnormal muscle posture and tension), urinary bladder muscle relaxation, esophageal sphincter muscle relaxation, and tics. However at the same time, the toxin released can cause botulism poisoning. Proteolytic strains of toxin is produced at around 35°C and for nonproteolytic strains, they can grow in environments of 26-28°C. Toxin produced from bacterium will cause botulism which is food poisoning that will lead to muscle paralysis. [25] It can also cause gas gangrene, systemic toxemia, shock, and mild enterotoxaemia in humans. [23] | |||
<br> | |||
====<b><I>Enterobacterium</I></b><br>==== | |||
Members of the genus Enterobacterium are gram-negative, straight rods, and sometimes motile species. Depends on types of species, they can grow at temperature range of 25-37 Celcius in such places at soil, water, plants and animails. They function as facultative anaerobic, oxidase-negative, glucose fermenters [10] and nitrate reducers. This family composed of more than 150 bacterial strains that consist mostly of <I>[[Escherichia coli]]</I>, <I>Klebsiella pneumonia</I>, <I>Klebsiella oxytococa</I>, and <I>Enterobacter cloaeces</I>. [29] Members belonging to the genera <I>Serratia</I>, <I>[[Enterobacter]]</I>, <I>Proteus</I> and <I>Hafnia</I> often contribute to meat spoilage. <I> Serratia</I> spp. can produce three enzymes such as lipase, DNase, and gelatinase. <I>Enterobacter</I> spp. can easily be grown in laboratory. They can ferment glucose when acid and gas are presented. <I>Hafnia alvei</I> is easily grown on media and motile. <I>Hafnia</I> spp. and <I>Proteus</I> spp. can resemble salmonella and can clump in polyvalent salmonella antisera. In addition, <I>Proteus</I> spp. can produce concentric zones if placed in blood agar. They also are resistant to polymycin B and colistin, similar to <I>Serratia</I> spp. Thus when these species are combined, they can bring changes to the meat composition. For example, in vacumm-packed spoiled meat, <I>Hafnia alvei</I> and <I>Serratia spp</I> are often capable of producing N-acyl homoserine lactones (AHLs), which activate the signal that control gene expression. [3] | |||
<br> | |||
< | ====<B><I>[[Leuconostoc]]</I></B><br>==== | ||
[[Image:Leuconostocwiki.jpg|right|100x200px|frame|Leuconostoc Culture]] | |||
<I>Leuconostoc</I> is one of the lactic acid bacteria; it produces D-lactate and ethanol. This group of microbe is responsible for the discoloration, gas production, and buttery smell of spoiled meat. [2] The genus <I>Leuconostoc</I> is described as being spherical cells that is gram-positive and often lenticular on agar. This bacterium grows optimally in an environment of 20-30°C and in modified atmospheres. However, they also require a rich and complex media for growth. A rich and complex media includes nicotinic acid, thiamin, biotin, and pantothenic acid. For energy, they are heterofermentatives, which means they use a combination of pentose phosphate and phosphoketolase pathways. This microbe cannot go through spore formation for survive. They fall under the facultative anaerobic category, which means they can live in an environment with or without oxygen. <I>Leuconostoc</I> was originally placed into <I>Streptococcaceae</I> bacteria family as mentioned in Bergey’s Manual of Determinative Bacteriology. [13] However, in 1986, the Bergey’s Manual of Systematic Bacteriology moved <I>Leuconostoc</I> from the <I>Streptococcaceae</I> family into the <I>Deinococcaceae</I> family. [13] The green spots on a slice of spoiled meat are caused by the H<sub>2</sub>O<sub>2</sub> created by <i>[[Leuconostoc]]</I>. <I>Leuconostoc mesenteroides</i>, <I>[[Leuconostoc carnosum]]</I>, and <I>Leuconostoc amelibiosum</I> are responsible for the accumulation of CO<sub>2</sub> production. [13] Aside from finding <I>Leuconostoc</I> in spoiled meat, it can also grow in plants, fermenting vegetables, milk, dairy products, wine, and even human blood. [13] | |||
<br> | <br> | ||
====<b><I>[[Pseudomonas]]</I></b><br>==== | |||
[[Image:Pseudomonas.jpg|right|frame|Pseudomonas]] | |||
The predominant bacteria that are often associated with spoiled meat are <I>Pseudomonas</i>. They are polarly flagellated, gram –negative, rod shaped, aerobic bacteria. [1]. A few microorganisms under the genus <i>Pseudomonas</I> are known to effectively use meat as a niche due to their ability to break down glucose and amino under aerobic conditions and at refrigerated temperature. <i>Pseudomonades</I> are able to break down the long peptide chains of proteins in meats into amino acids and foul-smelling compounds such as ammonia, amines, and hydrogen sulfide [11]. Some strain of <i>Pseudomonas</i> produce esters, many produce sulfur-containing compounds, and a few produce methyl ketones, secondary alcohols, and unsaturated hydrocarbons [7]. Florescent <i>Pseudomonas</i> strains represent one of the most important groups among <i>Pseudomonas</i> because of their ability to produce water-soluble yellow-green pigments, called pyoverdines (PVDs). These yellow-green pigments act as siderophores, allow <i>Pseudomonas</i> to uptake iron from their environment. The most common <i>Pseudomonas</i> species found in beef, pork, lamb and poultry meat appears to be <i>Pseudomonas fragi</i>. Perhaps <i>Pseudomonas fragi</I> strains are so dynamic because it is capable of using a wide range of carbon compounds including D-arabinose, creatine, and bile acids [19]. <i>Pseudomonas fragi</I> growing on meat surface uses compounds such as glucose, free amino acids, and lactate. These carbon sources are enough support growth until spoilage has occurred. When the concentration of these compounds decrease in the uppermost layer, the compounds diffuse from below. Proteolytic activity and penetration of bacteria down in the tissue does not occur before the meat is already spoiled. In general, <i>Pseudomonas</i> shows preference for glucose. It is only when glucose is depleted that the <i>Pseudomonas</i> takes up the free amino acids (the amino acids are consumed before lactate). The order of preference from most to least is glucose>lactate>citrate>aspirate-glutamate>creatine-creatinine. It is at the point when amino acids are consumed that the meat gives off an offensive odor from the volatile by-products of amino acid catabolism. [18]. | |||
<br> | |||
===Interactions between microbes=== | |||
Meat is a complex niche that has chemical and physical properties which allow the habitation and development of many and a variety of microorganisms. Many factors can influence which microbes are presence on certain meat. After slaughtering, meat can be contaminated with bacteria from the water, air, and soil as well as from the workers and the equipments involved during the manufacturing process. When different microbes live in the same environment, they are certain to interact with each other. However, in the study by Russo et al (2006), the microbes in question that was living on the same piece of meat have little signs of significant interactions. [22] When studying the interaction of <I>Brochothrix Thermosphacta</I> with <I>[[Pseudomonas]]</I> and <I>Enterobacteriaceae</I>, <I>Brochothrix Thermosphacta</I> show the same growth pattern as when growing alone. However, in the presence of Lactic Acid Bacteria (LAB), <I>Brochothrix Thermosphacta</I> shows a slower growth. No evidence suggests that The LAB population have antagonistic activity through bacteriocin production. The observed antagonistic activity must be due to the decrease in pH and increase competition for available substrate [22]. In another study by Gill and Newton, the researchers examined the interaction of paired pure cultures of <I>Pseudomonas spp.</I>, <I>Brochothrix thermosphacta</I> and <I>Enterobacterium spp.</I> on raw meat slices. Microbes could out-compete each other only when they reach the maximum cell density; otherwise, no interactions were observed. [9] For some microbes such as <I>[[Clostridium]]</I> can go through sporulation as a survival mechanism. [20] Another study done by Peneau (2007) also attempted to find out the relationship between microbes that grow in spoiled meat. For this study, the researchers focused on <I>[[Pseudonomas]]</I>, <I>[[Staphylococcus]]</I>, and <I>[[Listeria monocytogenes]]</I>. Results showed that some strains of <I>Pseudonomas</I> and <I>Staphylococcus</I> can increase the growth of <I>Listeria monocytogenes</I> and/or even help protect the bacterium from disinfectants. [21] This shows that the effect of one microbe might be beneficial to another microbe even though symbiosis does not occur. In a different study done by Metaxopoulos et al (2002), the lactic acid bacteria's interaction with other microbes was explored. This study showed that there might be some evidence of a little negative interaction. The presence of lactic acid bacteria has shown to prevent spoilage flora from growing. Although there is a slight inhibition in microbe growth, microbes are growing, which means the negative interaction is not very strong. But this study does show that researchers have yet to figure out how all the microbes involved with spoilage interact with each other. [16] There needs to be more research on the interactions between the different microbes. | |||
=== | ===Effects of microbes on their environment=== | ||
< | <I>Brochothrix thermosphacta</I>, <I>Carnobacterium spp.</I>, <I>Enterobacteriaceae</I>, <I>Lactobacillus spp.</I>, <I>Leuconostoc spp.</I>, <I>Pseudomonas spp.</I>, <I>Shewanella putrefaciens</I> and <I>Weissella spp.</I> all work together to give meat its spoiled characteristics: discoloration, gas production, slime production, decrease in pH, and sour off-flavor. [2] The change in pH can be an advantage for some microbes because that might be the optimal growing environment for them. The slime production might also be advantageous for some bacterium since some bacteria survive the best in moist environments. The slime can be used as a protective layer to keep the bacteria moist. | ||
<I>Leuconostoc</I> produces H<sub>2</sub>O<sub>2</sub>, which gives spoiled meat its green discoloration. [13] This discoloration will change the environment for other microbes because molecules are being oxidized. Some microbes may be able to utilize the oxidized form of the molecule while others may not. | |||
<I>Clostridium</I> work with lactic acid bacteria [<I>Lactobacillus</I> and <I>Leuconostoc</I>] to produce large amounts of gas (H<sub>2</sub> and CO<sub>2</sub>). Usually this is accompanied by a foul odor. [2] This increase in CO<sub>2</sub> gas will change the environment by providing bacterium with more gas that can be used for carbon cycling or other pathways. | |||
When pH is around 6.5 or higher and there is an increased concentration of glucose and alpha-ketoisocaproic acid, <I>C. maltaromaticum</I> strain would increase the leucine production of 3-methylbutanal, 3-methylbutanol and 3-methylbutanoic acid. This would result in the lower leucine concentration and cause an effect on odor. [15] | |||
===Metabolism microbes carry our that affects their environment=== | |||
<I> | <I>Clostridium</I> can perform nitrogen fixation. <I>Clostridium</I> can go through fermentation of carbon sources to produce acetone, butanol, ethanol, isopropanol, and organic acids. [20] | ||
<I> | <I>Leuconostoc</I> produces ammonia by the use to bacterial deamination of amino acid and the production of ammonia will lead to a decrease in acidity. The process it takes to produce H<sub>2</sub>O<sub>2</sub> involves the oxidation of nitrosohaemochrome to choleomyoglobin. [13] | ||
<I>Pseudomonas</I> and <I>Brochothrix thermosphacta</I> predominantly contribute to the odor that spoil meat gives off as result of their metabolism. The foul odor comes from compounds such as ammonia, amines, and hydrogen sulfide from the break proteins. <i>B. thermosphacta</i> aerobic metabolism of glucose produces foul-smelling odor such as acetoin and acetic acid. [14] | |||
<I> | <I>C. divergens</I> and <I>C. maltaromaticum</I> can use ribose and gluconic acids as substrates to produce acetic acid, formic acid and CO<sub>2</sub> via pyruvic acid decarboxylation. Under aerobic conditions, the amount of acetic acid the media can produce could exceed that of lactic acid production. During growth, <I>C. maltaromaticum</I> can also produce ethanol from glucose and ribose, and acetonim from pyruvic acid. [15] | ||
===Presence of non-microbes=== | |||
The main organism that contributes to spoiled meat are bacteria, most noticeably Pseudomonas. However, when antibiotic is used to suppress bacterial growth in meat, molds become the primary contributer of spoilage. The most common yeasts that are found in spoiled poultry are <i>Candida</i>, <i>Rhodotorula</i>, <i>Dehbaryomyces</i>, and <i>Yarrowiwa</i>. Signs of spoilage include sliminess at the surface of the meat. <i>Thamnidium </i>, <i>Mucor</i>, and <i>Rhizopus</i>, contributes of the appearance of “whiskers” on beef and <i>Cladosporium</i> is responsible for the “black spot”on meats. <I>Penicillium</I> produces green patches while <i>Sporotrichum</i> and <i>Chrysosporium</i> produce “white spot.” Molds usually do not grow on meat at temperature below 5°C. It tends to dominate the meat system when the meat surface is too dry for bacterial growth or when meat is treated with antibiotic such as tetracylines. The presence of bacteria virtually guaranteed that molds cannot grow there. It appears that bacteria grow faster than molds and so the bacteria would consume all the available surface oxygen that is require for molds functions.[6] | |||
== | ==Summary== | ||
Meat, as a ecological niche, is a vibrant place that is potentially a breeding ground for undesirable microorganisms, varying in characteristics and lifestyle. To minimize excess bacteria, it is important to handle meat accordingly. Certain protocol should be followed: Cook meat at a temperature of 65-75°C or 160°F, which kills most vegetative cells via heat. When storing, seal the meat in a modified atmosphere or vacuum when possible to prevent interactions with neighboring niches and communities of bacteria, and refrigerate at a temperature around 5°C to prevent spoilage bacteria from prospering.[2] | |||
==Current Research== | ==Current Research== | ||
2006: <I>Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: population analysis by rDNA-based methods</I><br> | 2006: <I>Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: population analysis by rDNA-based methods</I><br> | ||
The investigators aimed to research and find which lactic acid bacteria was involved | The investigators aimed to research and find which lactic acid bacteria was involved with the spoilage of vacuum packaged cooked meat products. They did this by studying different samples of bacteria within 4 meat products, some of which had spoilage symptoms, some that did not. Colonies of these were then grown on yeast glucose lactose peptone and trypticase soy yeast plates, and where then identified via internal spacer region. The study found that <i>Leuc. Mesenteroides</i> was the main spoilage agent within vacuum packaged meats. The significance of this study was to determine what organisms to look for to prevent the spoilage of vacuum packaged meats. [4] | ||
<br><br> | <br><br> | ||
2005: <i>Development of a Microbial Model for the Combined Effect of Temperature and pH on Spoilage of Ground Meat, and Validation of the Model under Dynamic Temperature Conditions</I><br> | 2005: <i>Development of a Microbial Model for the Combined Effect of Temperature and pH on Spoilage of Ground Meat, and Validation of the Model under Dynamic Temperature Conditions</I><br> | ||
The study aimed at using microbiological and sensory analysis to predict spoilage of aerobic stored ground meat. Under aerobic conditions, samples of ground meat (beef and pork) were analyzed for changes in their appearances, smells and microbes composition at certain ranges of pH (5.34-6.13) and temperature (0- | The study aimed at using microbiological and sensory analysis to predict spoilage of aerobic stored ground meat. Under aerobic conditions, samples of ground meat (beef and pork) were analyzed for changes in their appearances, smells and microbes composition at certain ranges of pH (5.34-6.13) and temperature (0-20°C). As observed, <i>Pseudomonads</i> were the predominant bacteria isolated from these samples. In addition, it was also detected that the changes in <i>Pseudonomads</i> populations is proportional to the sensory changes. Thus,it can be concluded that microbiological and sensory analysis can be used as a “good index" for determining spoilage of meat stored aerobically. Following this type of model, the meat industry can benefit from using more “effective management systems, which will optimize the quality of meat products”. [14]<br><br> | ||
2003: <I>In vitro and in situ growth characteristics and behaviour of spoilage organisms associated with anaerobically stored cooked meat products</I> <br> | 2003: <I>In vitro and in situ growth characteristics and behaviour of spoilage organisms associated with anaerobically stored cooked meat products</I> <br>[[Image:ham.jpg|right|frame|Ham]] | ||
This study was aimed to research the different types of spoilage affects caused by different | This study was aimed to research the different types of spoilage affects caused by different organisms, in vacuumed packaged cooked meat products. They did this by characterizing strains of different spoilage organisms in a 7°C anaerobic broth. The growth rate, acidifying character, and metabolite production was compared. Then the organisms were inoculated onto cooked meat, and characteristics were again observed, including spoilage, microbial growth, pH, metabolite production, and also sensory changes. The results concluded that the microbial organisms <I>B. thermosphacta</i> and <I>Leuconostoc mesenteroids</I> induced spoilage on cooked meat products the quickest. This study determined correlations between microbial growth, changes in pH and metabolite formation and various spoilage organisms upon cooked ham. [27] <br><br> | ||
2002: <I>Microbial interaction in cooked cured meat products under vacuum or modified atmosphere at 4º C</I><br> | 2002: <I>Microbial interaction in cooked cured meat products under vacuum or modified atmosphere at 4º C</I><br> | ||
This study was aimed to find out the antagonistic activity of the lactic acid bacteria strains Leuconostoc mesenteroides and Lactobacillus curvatus against spoilage in two types of cooked meat. The results concluded that the lactic acid did help prevent spoilage while they did not negatively affect the meat. The study found that biopreservation can help increase the shelf life of meat produce. [ | This study was aimed to find out the antagonistic activity of the lactic acid bacteria strains <i>Leuconostoc mesenteroides</i> and <i>Lactobacillus curvatus</i> against spoilage in two types of cooked meat. The results concluded that the lactic acid did help prevent spoilage while they did not negatively affect the meat. The study found that biopreservation can help increase the shelf life of meat produce. [16] | ||
==References== | ==References== | ||
[1] [http:// | [1] | ||
[http://ijs.sgmjournals.org/cgi/reprint/50/4/1563 Anzai Y., Kim H., Park, J., and Wakabayashi H. "Phylogenetic affiliation of the <I>Pseudomonads</I> based on 16S rRNA sequence". ''International Journal of System Evolution Microbiology''. 2000. Volume 50. p. 1563–89.] | |||
[2] | [2] | ||
[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7K-3RG566P-V&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=56e3f644f65f2f64ee0879034566c8e5 Borch, E., Kant-Muermans, M., and Blixt, Y. "Bacterial Spoilage of Meat and Cured Meat Products". ''International Journal of Food Microbiology". 1996. Volume 33. p. 103-120.] | [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7K-3RG566P-V&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=56e3f644f65f2f64ee0879034566c8e5 Borch, E., Kant-Muermans, M., and Blixt, Y. "Bacterial Spoilage of Meat and Cured Meat Products". ''International Journal of Food Microbiology". 1996. Volume 33. p. 103-120.] | ||
[3] | [3] | ||
[http://www.pubmedcentral.nih.gov/ | [http://www.pubmedcentral.nih.gov/picrender.fcgi?tool=pmcentrez&artid=444785&blobtype=pdf Bruhn, J., Christensen, A., Flodgaard, L., Nielsen, K., Larsen, T., Givskov, M., and Gram, L. “Presence of Acylated Homoserine Lactones (AHLs) and AHL-Producting Bacteria in Meat and Potential Role of AHL in Spoilage of Meat". ‘’Applied and Environmental Microbiology.’’ 2004. Volume 70. p. 4293-4302.] | ||
[4] | [4] | ||
[http:// | [http://www3.interscience.wiley.com/cgi-bin/fulltext/118490301/PDFSTART Chenoll, E., Macian, M., Elizaquivel, P., and Aznar, R. "Lactic Acid Bacteria Associated with Vacuum-packed Cooked Meat Product Spoilage: Population Analysis by rDNA-based Methods". ''Journal of Applied Microbiology''. 2006. Volume 102. p. 498-508.] | ||
[5] | [5] | ||
[http:// | [http://www3.interscience.wiley.com/cgi-bin/fulltext/120054845/PDFSTART Dainty, R. and Christine M. Hibbard “Aerobic Metabolism of Brochothrix Thermosphacta Growing on Meat Surface and in Laboratory Media”. ‘’Journal of Applied Microbiology’’. 1980. Volume 48. No 3. p. 387-396.] | ||
[6] | [6] | ||
[http:// | [http://springerlink.metapress.com/content/v7244233753p5wq0/fulltext.pdf Davies, A. and Board R. “Fresh Meat and Poultry”. ‘’The Microbiology of Meat and Poultry’’. 1998. p. 63-99.] | ||
[7] | [7] | ||
[http://www.ncbi.nlm.nih.gov/pubmed/3610888 Edwards, R., Dainty, R., and Hibbard, C. “Volatile Compounds Produced by Meat <I>Pseudomonads</I> and Related Reference Strains”. ‘’Journal of Applied Bacteriology’’. 1987. Volume 36. p. 403-412.] | |||
[8] | [8] | ||
[http://aem.asm.org/cgi/reprint/72/7/4663 Ercolini, D., Russo, F., Torrieri, E., Masi, P., and Villani, F. “ Changes in the Spoilage- Related Microbiota of Beef During Refrigerated Storage Under Different Packaging Conditions". ''American Society for Microbiology''. 2006. Volume 72. p. 4663-4671.] | |||
[9] | [9] | ||
[http://www.ncbi.nlm.nih.gov/sites/entrez Gill, C., and Newton, K. “The Development of Aerobic Spoilage Flora on Meat Stored at Chill Temperatures”. ‘’Journal of Applied Bacteriology’’. 1977. Volume 43. p. 189–195.] | |||
[10] | [10] | ||
[http:// | [http://www.hpa-standardmethods.org.uk/pdf_sops.asp. Health Protection Agency. "Identification of <I>Enterobacteriaceae</I>'. ''National Standard Method''. 2007. BSOP ID 16: Issue 2.] | ||
[11] | [11] | ||
[http://www3.interscience.wiley.com/cgi-bin/fulltext/ | [http://www3.interscience.wiley.com/cgi-bin/fulltext/120078961/PDFSTART Ingram, M. and Dainty, R. “Changes Caused by Microbes in Spoilage of Meats”. ‘’Journal of Apply Microbiology’’. 1971. Volume 34. No 1. p. 21-39.] | ||
[12] | [12] | ||
[http:// | [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7K-4691HKR-3&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=4f6a6676630f8677d08f733735be9147 Jay, J., Vilai, J., and Hughes, M. “Profile and activity of the bacterial biota of ground beef held from freshness to spoilage at 5-7ºC". ''International Journal of Food Microbiology''. 2003. Volume 81. p. 105-111.] | ||
[13] | [13] | ||
[http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=11792842 Shimizu, T., Ohtani, K., Hirakawa, H., Ohshima, K., Yamashita, A., Shiba, T., Ogasawara, N., Hattori, M., Kuhara, S., and Hayashi, H. “Complete Genome Sequence of i>Clostridium perfringens</I>, an Anaerobic Flesh-Eater”. ‘’Proceedings of National Academy of Sciences of the United States of America’’. 2002. Volume 99. No 2. p. 996-1001.] | [http://www.ncbi.nlm.nih.gov/pubmed/16085830?ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum Koort, J., Murros, A., Coenye, T., Eerola, S., Vandamme, P., Sukura, A., and Bjorkroth, J. “<I>Lactobacillus oligofermentans sp.</I> nov., Associated with Spoilage Modified-Atmosphere-Packaged Poultry Products”. ''Applied and Environmental Microbiology''. 2005. Volume 71. No 8. p. 4400-4406] | ||
[14] | |||
[http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1352189 Koutsoumanis, K., A. Stamatiou, P. Skandamis, and G.-J. E. Nychas. "Development of a Microbial Model for the Combined Effect of Temperature and pH on Spoilage of Ground Meat, <I>Leuconostocs</I>''. ''Journal of Dairy Science''. 1995. Volume 78. no. 11. P. 2514-2522] | |||
[15] | |||
[http://www3.interscience.wiley.com/cgi-bin/fulltext/118494489/PDFSTART Leisner, J., Laursen, B., Prevost, H., Drider, D., and Dalgaard, P. "<I>Carnobacterium</I>: Positive and Negative Effects in the Environment and in Foods". ''Federation of European Microbiological Societies Microbiology Review''. 2007. Volume 31 p. 592-613.] | |||
[16] | |||
[http://www3.interscience.wiley.com/cgi-bin/fulltext/118961890/PDFSTART Metaxopoulos, J., Mataragas, M., and Drosino, E. "Microbial interaction in cooked cured meat products under vacuum or modified atmosphere at 4º C". ''Journal of Applied Microbiology''. 2002. Volume 93. p. 363-373] | |||
[17] | |||
Meyer, J., Halle, F., Hohnade1l, D., Lemanceau, P., and Ratefiarivelo, | |||
H. “Siderophores of Pseudomonas ± Biological Properties in Iron Transport in Microbes, Plants and Animals”. 1987. p. 188-205. p. 363-373.] | |||
[18] | |||
[http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=241744 Molin, G. “Mixed Carbon Source Utilization of Meat-Spoiling <I>Pseduomonas fragi</I>”. ‘’Applied and Environmental Microbiology’’. 1985. Volume 49. No 6. p. 1442-1447.] | |||
[19] | |||
[http://ijs.sgmjournals.org/cgi/reprint/36/2/257?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&fulltext=Phenotypically+Based+Taxonomy+of+Psycholtrophic+Pseudomonas+Isolated+from+Spoile&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT Molin, G., and Ternstrom, A. “Phenotypically Based Taxonomy of Psycholtrophic <I>Pseudomonas</I> Isolated from Spoiled Meat, Water, and Soil”. ‘’International Journal of Systematic Bacteriology’’. 1986. Volume 36. No 2. p. 257-274.] | |||
[20] | |||
[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=PubMed&cmd=Retrieve&list_uids=11466286&dopt=Citation Nolling J., Breton G., Omelchenko M.V., Makarova K. S., Zeng Q., Gibson R., Lee H. M., Dubois J., Qui D., Hitti J., Wolf Y. I., Tatusov R. L., Sabathe F., Doucette-Stamm L., Soucaille P., Daly M.J., Bennett G.N., Koonin E. V., and Smith D. R. "Genome sequence and comparative analysis of the solvent-producing bacterium <I>Clostridium acetobutylicum</I>". ''Journal of Bacteriology''. 2001 Volume 183. no. 16. p. 4823-38.] | |||
[21] | |||
[http://aem.asm.org/cgi/reprint/73/9/2839 Peneau, S., Chassaing, D., and Carpentier, B. “First Evidence of Division and Accumulation of Viable but Nonculturable <I>Pseudomonas fluorescens</I> Cells on Surfaces Subjected to Conditions Encountered at Meat Processing Premises”. ‘’Applied and Environmental Microbiology’’. 2007. Volume 73. No 9. p. 2839-2846] | |||
[22] | |||
[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFP-4JS1MVM-1&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=cdc21aba630cfedde626bdbee5be0b27 Russo, F., Ercolini, D., Mauriello, G., and Villani, F. “Behavior of Brochothrix Thermosphacta in Presence of Other Meat Spoilage Microbial groups”. ‘’Food Microbiology’’. 2006. Volume 23. No 8. p. 797-802.] | |||
[23] | |||
[http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=11792842 Shimizu, T., Ohtani, K., Hirakawa, H., Ohshima, K., Yamashita, A., Shiba, T., Ogasawara, N., Hattori, M., Kuhara, S., and Hayashi, H. “Complete Genome Sequence of <i>Clostridium perfringens</I>, an Anaerobic Flesh-Eater”. ‘’Proceedings of National Academy of Sciences of the United States of America’’. 2002. Volume 99. No 2. p. 996-1001.] | |||
[24] | |||
Stackebrant, E. and Jones D. “The Genus <I>Brochothrix</I>”. ‘’The Prokaryotes’’. 2006. p. 447-491. | |||
[25] | |||
[http://www.fda.gov/fdac/features/095_bot.html Vangelova, L. “Botulinum Toxin: A Poison That Can Heal”. ''FDA Consumer Magazine''. 1995.] | |||
[ | [26] | ||
[http:// | [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7K-4CP14B0-1&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=f906ed193c5e8287c37fccad2a30c34d Vermeiren, L., Devlieghere, F., and Dbevere, J. “Evaluation of meat born lactic acid bacteria as protective culture for the biopreservation of cooked meat products”. ‘’International Journal of Food Microbiology’’. 2004. Volume 96. p. 149-164.] | ||
[ | [27] | ||
[http:// | [http://www3.interscience.wiley.com/cgi-bin/fulltext/118711191/PDFSTART Vermeiren, L., Devlieghere, F., De Graef, V., and Debevere, J. "In vitro and in situ growth characteristics and behaviour of spoilage organisms associated with anaerobically stored cooked meat products". ''Journal of Applied Microbiology''. 2003. Volume 98. p. 33-42.] | ||
[ | [28] | ||
[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7K-4PJM9DS-4&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=8a8a3f5c8f66bb833f662cf781918f33 Vihavainen, E., and Björkroth, K. “Spoilage of value-added, high-oxygen modified-atmosphere packaged raw beef steaks by <I>Leuconostoc gasicomitatum</I> and <I>Leuconostoc gelidum</I>'. ''International Journal of Food Microbiology''. 2007. Volume 119. p. 340-345.] | [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T7K-4PJM9DS-4&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=8a8a3f5c8f66bb833f662cf781918f33 Vihavainen, E., and Björkroth, K. “Spoilage of value-added, high-oxygen modified-atmosphere packaged raw beef steaks by <I>Leuconostoc gasicomitatum</I> and <I>Leuconostoc gelidum</I>'. ''International Journal of Food Microbiology''. 2007. Volume 119. p. 340-345.] | ||
[ | [29] | ||
[http:// | [http://jcm.asm.org/cgi/reprint/45/4/1167 Weigand, I., Geiss, H., Mack, D., Sturenburg, E., and Seifert, H. "Detection of Extended-Spectrum Beta-Lactamases among <I>Enterobacteriaceae</I> by Use of Semiautomated Microbiology Systems and Manual Detection Procedures". ''Journal of Clinical Microbiology''. 2007. Volume 45 p. 1167-1174.] | ||
[30] | |||
"Spoiled Meat". Spoiled Meat Niche Group's Image. 2008. | |||
Edited by | Edited by Steven Lee, Jade Nguyen, Sarah Paek, June Tse, Amy Vo, students of [mailto:ralarsen@ucsd.edu Rachel Larsen] | ||
Latest revision as of 03:03, 20 August 2010
Introduction to Spoiled Meat
Meat is a complex niche that has chemical and physical properties which allow the colonization and development of a variety of microorganisms. Many factors can influence which microbes are present on certain meat. After slaughtering, meat can be contaminated with bacteria from the water, air, and soil as well as from the workers and the equipments involved during the manufacturing process. The point of spoilage is normally defined as the maximum acceptable bacterial level, the unacceptable odor/flavor, or the appearance for consumption. [2] However, the common features of spoilage consist of discoloration, gas production, and foul odor. Most of the organisms that live within this niche lives in an atmosphere of a pH under 7 and around 4-7°C. The living conditions might change which will determine the rate of organism production. Although there are many different types of meat, most of the organisms that grow in this niche are similar. This page will focus on the three most common sources of meat: pork, chicken, and beef. Spoiled meat varies in different forms, from being raw, marinated, or being cooked and cured. The spoiled meat is stored in a vacuum or modified atmosphere.[16]
Description of Niche
Physical Conditions
Because of its significant nutrients available on the surface and its high water content, meat is one of the most perishable foods. The conditions of spoiled meat vary on the type of meat, how the meat was prepared, and how the meat is stored.
Pork
The follow conditions are for Frankfurter-type sausages and sliced pork. For meat that is initially cooked/heated at 65-75º C [26] and stored in a vacuum or modified atmosphere, at a temperature of 4º C, the salt content is around 2%, with a pH of above 6, making it a generally neutral environment. The moisture is 61.5% in Frankfurter-type sausages and 68% in sliced pork [16].
Chicken
Marinated chicken meat in a modified atmosphere (MA) packaged and stored at temperatures ranging from 3.4 to 7.7ºC. The marinating factor includes many sub-ingredients such as acidic sauce including sucrose or glucose, salt, and spices that adds to the flavor of meat. [13] The marinate provides an acidic pH of 4.7 to 5.0. [13]
Beef
For ground beef (fresh from the market) stored aerobically (high oxygen MA) at the temperature of 5-7ºC, chilled- refrigerated, the pH was 5.5 to 5.92. [12] For raw beef steaks, the pH was 5.4 to 5.6 the lactic acid bacteria level was 2.2 × 104 CFU/g [28]. The beef steak meat packages were also moisture enhanced with levels between 112 and 115% of original weight [28].
Influence by Adjacent Communities
Because it is stored in a modified, vacuum, refrigerated atmosphere, the spoiled meat niche is not close to another niche, but the handling and process of packaging meat and can often add microflora to the products. An estimated .5-2 log CFU/g of bacteria is added to the meat due to these processes. [26]
Conditions under which the environment changes
The influenced on spoiled meat is by the microflora on the meat itself, mainly Lactobacillus sp. and Leuconostoc sp., and human added Lactic acid bacteria. Lactic acid bacteria are antagonistic cultures added to the meat in order to inhibit pathogens and increase the shelf life, which are protective cultures as a means of biopreservation. In this way, Lactic acid changes the condition of the niche by inhibiting unwanted microorganisms, via several methods, such as the production of bacteriocins and enzymes, and also by simply competing for nutrients in the same niche.
The niche community changes as the shelf life continues. If stored properly, either refrigerated or in an anaerobic atmosphere, lactic acid bacteria can prevent microflora from spoiling the meat too quickly, and this will allow other microbes to participate in the spoilage process. [26] As the shelf life reaches its limit, Lactic acid bacteria along with other bacterial populations increase which causes spoilage. Depending on the product handling after cooking, the spoiled pork may be recontaminated with up to 0.5-2 log CFU/g of total bacteria. [26]
In frankfurter-type sausages, the presence of Lact. curvatus can potentially change the physical conditions of the niche by decreasing the pH of the spoiled meat (from 6.6 to 5.8) at the end of its shelf life, after 28 days. In pork, the presence of Lactic Acid bacteria caused a decrease of pH from 6.6 to 5.1-5.3 (vacuum) and 5.5-5.6 (modified atmosphere) at the end of its shelf life. [16]
The conditions of the niche consists can cause various changes ranging from souring, changing the flavor, texture, and color, producing gas and slime, and change in the pH level. Spoilage affects can be noticed at various points in the shelf life, as early as a few days and up to the end of the shelf life (18 to 42 days), where the meat is dominated by spoilage. [4] Meat often shows a distinct color change from a reddish meat color to a brown pigment and shows bulging due to gas formation after packaging. [8] Spoilage is also apparent by swelling of packages and characterized by a green coloring due to production of hydrogen peroxide [28] with a strong buttery odor due to putrescine and cadaverine. [13] In addition, samples of meat loses water during storage (weight loss). Slime formation may occur, such as biofilms at low temperatures and have quorum sensing which also produces pigment changes that may not be suitable for human consumption. The development of spoilage is also associated to microbial consumption of meat nutrients such as sugars and amino acids. [8]
The influence of Chemicals
Increasing concentrations of CO2, a decreased pH, and a chilled storage are used to preserve meat and prevent spoilage microflora from forming [12]. But still, the dominant organisms Lactobacillus oligofermentans and Pseudomonas are present.
Marinating chicken often maintains the low pH because of the low pH of the marinate. The process of marinating in chicken can support the growth of Lactobacillus oligofermentans in which its population can grow up to 108 to 1010 CFU/g. [13] Ingredients also used to promote flavoring and taste of beef/steak and vacuum packaging with glucose and sugar can expedite the spoilage process that is depicted through a green coloring and a strong buttery odor of the meat. [28]
In the experiment Spoilage of value-added, high-oxygen modified-atmosphere packaged raw beef steaks by Leuconostoc gasicomitatum and Leuconostoc gelidum, the effects of O2 and CO2 content was studied via three packaging conditions of beef. All conditions were modified atmosphere packaged but with different concentrations of O2 and CO2: MAP1 consists of only air; MAP2 is 60% O2 and 40% CO2; and MAP3 is 20% O2 and 40% CO2. [8] These three varying packaging conditions show different effects on the spoiling piece of meat. The conditions for MAP2 protect the spoilage of meat (little color change of the beef and little microbial loads) after refrigerated storage compared to MAP1 and MAP3. According to the findings of different packaging condition studies, many different changes occurred. For MAP1 where only oxygen is present, the concentration of O2 decreased from 21% to 0% and concentrations of CO2 increased from 0% to 25% in 14 days.[8] In the MAP2 condition, both concentrations of O2 and CO2 remain constant. [8] For MAP3, the same observations is seen for the first seven days, but during the next seven days (day 7-14), a drop in oxygen concentration and an increase in CO2 concentration is detected. [8]
Who lives there?
Meat provides a suitable niche for many microorganisms because it has a rich source of nutrients.The main microbes that are responsible for meat spoilage consist of Brochotrix thermosphacta, Carnobacterium, Clostridium, Enterobacterium, Leuconostoc, and Pseudomonas.
Microbes Present
Brochothrix thermosphacta
Brochothrix Thermosphacta is a microorganism for which meat is considered an ecological niche. Its ability to grow under both aerobic and anaerobic conditions makes a significant meat colonizer. The genus Brochothrix is characterizes as gram-positive, non-spore forming, non-motile, catalase-positive, facultatively anaerobic, regular rod shaped bacteria. The optimal temperature for growth is 20-25º C. The optimal pH for B. thermosphacta to grow is pH 7.0 but growth is seen within the ranges of pH 5-9. Brochothrix thermosphacta is more resistant to irradiation than common meat spoilage organism such as Pseudomonas but are affected by irradiation does of 0.5 to 2.0 kilogray. The species have often been isolated from irradiated meat and poultry. Although, they are an important spoilage organism found prepacked meats and in meat stored in chill temperature, they can also inhabit other niches such frozen foods, milk, and cream. Storage conditions often selectively favor its growth. Brochothrix ssp can grow at temperatures a low as 0ºC and under conditions of low oxygen concentration and high C02 concentration. For metabolism, Brochothrix thermosphacta has enzymes for both the hexose-monophosphate and glycolysis pathways of glucose. Fermentative metabolism of glucose always results in the production of L+ lactic acid, but other end products depend on growth conditions [24]. Major end products of aerobic metabolism of glucose by B. Thermosphacta growing on meat are acetoin and acetic, isobutyric, isovaleric and 2 methylbutyric acid. In minimal medium, glucose is the source of all the end products; However, in complex medium such as meat, only acetoin and acetic acid are derived from glucose; Isobutyric, isovaleric, and 2-methylbutyric acids are produced from valine, leucine, and isoleucine, respectively[5]. These compounds, or their derivatives, are responsible for the odor that often characterizes spoiled meat. Unlike proteolytic spoilage bacteria such as Pseudomonas, B. thermosphacta is usually found only on the meat surface. In prepacked meat, it grows in the area between the meat-plastic film [24].
Carnobacterium
Carnobacterium (C.) is gram-positive genus and contains nine species: C. divergens, C. maltaromaticum, C altherfunditum, C. funditum, C. gallinarum, C. mobile, C. inhibens, C. pleistocenium, and C. viridans. However, only C. divergens and C. maltaromaticium can frequently be isolated in meat products. In food, they are often found responsible for producing antimicrobial peptides and bacteriocin. C. divergens and C. maltaromaticum grow anaerobically at high CO2 concentration, low concentration and high pressure. They are able to metabolize arginine and other carbohydrates, which may contribute to their adjustment to the environment. Their catabolic activities were observed to play a role in sensory spoilage of meat products. The other seven species are not commonly encountered. One example of its effects on food is that, C. divergens can produce H2O2, and when it encounters C. vriridans, it would result in a green discoloration of ham. However, this genus has not been fully understood. Its full genome sequence has yet been determined. Thus more studies are needed for a full understanding of Carnobacterium effects in food. [15]
Clostridium
Clostridium is a rod-shaped cell with a gram-positive membrane. These microbes are anaerobes and some are toxin-producing pathogens. These pathogens include Clostridium difficile, Clostridium botulinum, Clostridium tetani, and Clostridium perfringens. Some of them produce acetone, butanol, ethanol, isopropanol, and organic acids. This bacterium can go through spore formation for survival. Clostridium produces large amounts of gas in packaged meat. It is usually coupled up with foul odors and causes the package to appear in a blown pack. [20] Aside from finding this bacterium in spoiled meat, it can also be found in soil, sewage, and animal intestines. The toxin produced by this bacterium can do harm and help heal. So far this toxin has helped treat dystonias (neurological diseases involved abnormal muscle posture and tension), urinary bladder muscle relaxation, esophageal sphincter muscle relaxation, and tics. However at the same time, the toxin released can cause botulism poisoning. Proteolytic strains of toxin is produced at around 35°C and for nonproteolytic strains, they can grow in environments of 26-28°C. Toxin produced from bacterium will cause botulism which is food poisoning that will lead to muscle paralysis. [25] It can also cause gas gangrene, systemic toxemia, shock, and mild enterotoxaemia in humans. [23]
Enterobacterium
Members of the genus Enterobacterium are gram-negative, straight rods, and sometimes motile species. Depends on types of species, they can grow at temperature range of 25-37 Celcius in such places at soil, water, plants and animails. They function as facultative anaerobic, oxidase-negative, glucose fermenters [10] and nitrate reducers. This family composed of more than 150 bacterial strains that consist mostly of Escherichia coli, Klebsiella pneumonia, Klebsiella oxytococa, and Enterobacter cloaeces. [29] Members belonging to the genera Serratia, Enterobacter, Proteus and Hafnia often contribute to meat spoilage. Serratia spp. can produce three enzymes such as lipase, DNase, and gelatinase. Enterobacter spp. can easily be grown in laboratory. They can ferment glucose when acid and gas are presented. Hafnia alvei is easily grown on media and motile. Hafnia spp. and Proteus spp. can resemble salmonella and can clump in polyvalent salmonella antisera. In addition, Proteus spp. can produce concentric zones if placed in blood agar. They also are resistant to polymycin B and colistin, similar to Serratia spp. Thus when these species are combined, they can bring changes to the meat composition. For example, in vacumm-packed spoiled meat, Hafnia alvei and Serratia spp are often capable of producing N-acyl homoserine lactones (AHLs), which activate the signal that control gene expression. [3]
Leuconostoc
Leuconostoc is one of the lactic acid bacteria; it produces D-lactate and ethanol. This group of microbe is responsible for the discoloration, gas production, and buttery smell of spoiled meat. [2] The genus Leuconostoc is described as being spherical cells that is gram-positive and often lenticular on agar. This bacterium grows optimally in an environment of 20-30°C and in modified atmospheres. However, they also require a rich and complex media for growth. A rich and complex media includes nicotinic acid, thiamin, biotin, and pantothenic acid. For energy, they are heterofermentatives, which means they use a combination of pentose phosphate and phosphoketolase pathways. This microbe cannot go through spore formation for survive. They fall under the facultative anaerobic category, which means they can live in an environment with or without oxygen. Leuconostoc was originally placed into Streptococcaceae bacteria family as mentioned in Bergey’s Manual of Determinative Bacteriology. [13] However, in 1986, the Bergey’s Manual of Systematic Bacteriology moved Leuconostoc from the Streptococcaceae family into the Deinococcaceae family. [13] The green spots on a slice of spoiled meat are caused by the H2O2 created by Leuconostoc. Leuconostoc mesenteroides, Leuconostoc carnosum, and Leuconostoc amelibiosum are responsible for the accumulation of CO2 production. [13] Aside from finding Leuconostoc in spoiled meat, it can also grow in plants, fermenting vegetables, milk, dairy products, wine, and even human blood. [13]
Pseudomonas
The predominant bacteria that are often associated with spoiled meat are Pseudomonas. They are polarly flagellated, gram –negative, rod shaped, aerobic bacteria. [1]. A few microorganisms under the genus Pseudomonas are known to effectively use meat as a niche due to their ability to break down glucose and amino under aerobic conditions and at refrigerated temperature. Pseudomonades are able to break down the long peptide chains of proteins in meats into amino acids and foul-smelling compounds such as ammonia, amines, and hydrogen sulfide [11]. Some strain of Pseudomonas produce esters, many produce sulfur-containing compounds, and a few produce methyl ketones, secondary alcohols, and unsaturated hydrocarbons [7]. Florescent Pseudomonas strains represent one of the most important groups among Pseudomonas because of their ability to produce water-soluble yellow-green pigments, called pyoverdines (PVDs). These yellow-green pigments act as siderophores, allow Pseudomonas to uptake iron from their environment. The most common Pseudomonas species found in beef, pork, lamb and poultry meat appears to be Pseudomonas fragi. Perhaps Pseudomonas fragi strains are so dynamic because it is capable of using a wide range of carbon compounds including D-arabinose, creatine, and bile acids [19]. Pseudomonas fragi growing on meat surface uses compounds such as glucose, free amino acids, and lactate. These carbon sources are enough support growth until spoilage has occurred. When the concentration of these compounds decrease in the uppermost layer, the compounds diffuse from below. Proteolytic activity and penetration of bacteria down in the tissue does not occur before the meat is already spoiled. In general, Pseudomonas shows preference for glucose. It is only when glucose is depleted that the Pseudomonas takes up the free amino acids (the amino acids are consumed before lactate). The order of preference from most to least is glucose>lactate>citrate>aspirate-glutamate>creatine-creatinine. It is at the point when amino acids are consumed that the meat gives off an offensive odor from the volatile by-products of amino acid catabolism. [18].
Interactions between microbes
Meat is a complex niche that has chemical and physical properties which allow the habitation and development of many and a variety of microorganisms. Many factors can influence which microbes are presence on certain meat. After slaughtering, meat can be contaminated with bacteria from the water, air, and soil as well as from the workers and the equipments involved during the manufacturing process. When different microbes live in the same environment, they are certain to interact with each other. However, in the study by Russo et al (2006), the microbes in question that was living on the same piece of meat have little signs of significant interactions. [22] When studying the interaction of Brochothrix Thermosphacta with Pseudomonas and Enterobacteriaceae, Brochothrix Thermosphacta show the same growth pattern as when growing alone. However, in the presence of Lactic Acid Bacteria (LAB), Brochothrix Thermosphacta shows a slower growth. No evidence suggests that The LAB population have antagonistic activity through bacteriocin production. The observed antagonistic activity must be due to the decrease in pH and increase competition for available substrate [22]. In another study by Gill and Newton, the researchers examined the interaction of paired pure cultures of Pseudomonas spp., Brochothrix thermosphacta and Enterobacterium spp. on raw meat slices. Microbes could out-compete each other only when they reach the maximum cell density; otherwise, no interactions were observed. [9] For some microbes such as Clostridium can go through sporulation as a survival mechanism. [20] Another study done by Peneau (2007) also attempted to find out the relationship between microbes that grow in spoiled meat. For this study, the researchers focused on Pseudonomas, Staphylococcus, and Listeria monocytogenes. Results showed that some strains of Pseudonomas and Staphylococcus can increase the growth of Listeria monocytogenes and/or even help protect the bacterium from disinfectants. [21] This shows that the effect of one microbe might be beneficial to another microbe even though symbiosis does not occur. In a different study done by Metaxopoulos et al (2002), the lactic acid bacteria's interaction with other microbes was explored. This study showed that there might be some evidence of a little negative interaction. The presence of lactic acid bacteria has shown to prevent spoilage flora from growing. Although there is a slight inhibition in microbe growth, microbes are growing, which means the negative interaction is not very strong. But this study does show that researchers have yet to figure out how all the microbes involved with spoilage interact with each other. [16] There needs to be more research on the interactions between the different microbes.
Effects of microbes on their environment
Brochothrix thermosphacta, Carnobacterium spp., Enterobacteriaceae, Lactobacillus spp., Leuconostoc spp., Pseudomonas spp., Shewanella putrefaciens and Weissella spp. all work together to give meat its spoiled characteristics: discoloration, gas production, slime production, decrease in pH, and sour off-flavor. [2] The change in pH can be an advantage for some microbes because that might be the optimal growing environment for them. The slime production might also be advantageous for some bacterium since some bacteria survive the best in moist environments. The slime can be used as a protective layer to keep the bacteria moist.
Leuconostoc produces H2O2, which gives spoiled meat its green discoloration. [13] This discoloration will change the environment for other microbes because molecules are being oxidized. Some microbes may be able to utilize the oxidized form of the molecule while others may not.
Clostridium work with lactic acid bacteria [Lactobacillus and Leuconostoc] to produce large amounts of gas (H2 and CO2). Usually this is accompanied by a foul odor. [2] This increase in CO2 gas will change the environment by providing bacterium with more gas that can be used for carbon cycling or other pathways.
When pH is around 6.5 or higher and there is an increased concentration of glucose and alpha-ketoisocaproic acid, C. maltaromaticum strain would increase the leucine production of 3-methylbutanal, 3-methylbutanol and 3-methylbutanoic acid. This would result in the lower leucine concentration and cause an effect on odor. [15]
Metabolism microbes carry our that affects their environment
Clostridium can perform nitrogen fixation. Clostridium can go through fermentation of carbon sources to produce acetone, butanol, ethanol, isopropanol, and organic acids. [20]
Leuconostoc produces ammonia by the use to bacterial deamination of amino acid and the production of ammonia will lead to a decrease in acidity. The process it takes to produce H2O2 involves the oxidation of nitrosohaemochrome to choleomyoglobin. [13]
Pseudomonas and Brochothrix thermosphacta predominantly contribute to the odor that spoil meat gives off as result of their metabolism. The foul odor comes from compounds such as ammonia, amines, and hydrogen sulfide from the break proteins. B. thermosphacta aerobic metabolism of glucose produces foul-smelling odor such as acetoin and acetic acid. [14]
C. divergens and C. maltaromaticum can use ribose and gluconic acids as substrates to produce acetic acid, formic acid and CO2 via pyruvic acid decarboxylation. Under aerobic conditions, the amount of acetic acid the media can produce could exceed that of lactic acid production. During growth, C. maltaromaticum can also produce ethanol from glucose and ribose, and acetonim from pyruvic acid. [15]
Presence of non-microbes
The main organism that contributes to spoiled meat are bacteria, most noticeably Pseudomonas. However, when antibiotic is used to suppress bacterial growth in meat, molds become the primary contributer of spoilage. The most common yeasts that are found in spoiled poultry are Candida, Rhodotorula, Dehbaryomyces, and Yarrowiwa. Signs of spoilage include sliminess at the surface of the meat. Thamnidium , Mucor, and Rhizopus, contributes of the appearance of “whiskers” on beef and Cladosporium is responsible for the “black spot”on meats. Penicillium produces green patches while Sporotrichum and Chrysosporium produce “white spot.” Molds usually do not grow on meat at temperature below 5°C. It tends to dominate the meat system when the meat surface is too dry for bacterial growth or when meat is treated with antibiotic such as tetracylines. The presence of bacteria virtually guaranteed that molds cannot grow there. It appears that bacteria grow faster than molds and so the bacteria would consume all the available surface oxygen that is require for molds functions.[6]
Summary
Meat, as a ecological niche, is a vibrant place that is potentially a breeding ground for undesirable microorganisms, varying in characteristics and lifestyle. To minimize excess bacteria, it is important to handle meat accordingly. Certain protocol should be followed: Cook meat at a temperature of 65-75°C or 160°F, which kills most vegetative cells via heat. When storing, seal the meat in a modified atmosphere or vacuum when possible to prevent interactions with neighboring niches and communities of bacteria, and refrigerate at a temperature around 5°C to prevent spoilage bacteria from prospering.[2]
Current Research
2006: Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: population analysis by rDNA-based methods
The investigators aimed to research and find which lactic acid bacteria was involved with the spoilage of vacuum packaged cooked meat products. They did this by studying different samples of bacteria within 4 meat products, some of which had spoilage symptoms, some that did not. Colonies of these were then grown on yeast glucose lactose peptone and trypticase soy yeast plates, and where then identified via internal spacer region. The study found that Leuc. Mesenteroides was the main spoilage agent within vacuum packaged meats. The significance of this study was to determine what organisms to look for to prevent the spoilage of vacuum packaged meats. [4]
2005: Development of a Microbial Model for the Combined Effect of Temperature and pH on Spoilage of Ground Meat, and Validation of the Model under Dynamic Temperature Conditions
The study aimed at using microbiological and sensory analysis to predict spoilage of aerobic stored ground meat. Under aerobic conditions, samples of ground meat (beef and pork) were analyzed for changes in their appearances, smells and microbes composition at certain ranges of pH (5.34-6.13) and temperature (0-20°C). As observed, Pseudomonads were the predominant bacteria isolated from these samples. In addition, it was also detected that the changes in Pseudonomads populations is proportional to the sensory changes. Thus,it can be concluded that microbiological and sensory analysis can be used as a “good index" for determining spoilage of meat stored aerobically. Following this type of model, the meat industry can benefit from using more “effective management systems, which will optimize the quality of meat products”. [14]
2003: In vitro and in situ growth characteristics and behaviour of spoilage organisms associated with anaerobically stored cooked meat products
This study was aimed to research the different types of spoilage affects caused by different organisms, in vacuumed packaged cooked meat products. They did this by characterizing strains of different spoilage organisms in a 7°C anaerobic broth. The growth rate, acidifying character, and metabolite production was compared. Then the organisms were inoculated onto cooked meat, and characteristics were again observed, including spoilage, microbial growth, pH, metabolite production, and also sensory changes. The results concluded that the microbial organisms B. thermosphacta and Leuconostoc mesenteroids induced spoilage on cooked meat products the quickest. This study determined correlations between microbial growth, changes in pH and metabolite formation and various spoilage organisms upon cooked ham. [27]
2002: Microbial interaction in cooked cured meat products under vacuum or modified atmosphere at 4º C
This study was aimed to find out the antagonistic activity of the lactic acid bacteria strains Leuconostoc mesenteroides and Lactobacillus curvatus against spoilage in two types of cooked meat. The results concluded that the lactic acid did help prevent spoilage while they did not negatively affect the meat. The study found that biopreservation can help increase the shelf life of meat produce. [16]
References
[17] Meyer, J., Halle, F., Hohnade1l, D., Lemanceau, P., and Ratefiarivelo, H. “Siderophores of Pseudomonas ± Biological Properties in Iron Transport in Microbes, Plants and Animals”. 1987. p. 188-205. p. 363-373.]
[24] Stackebrant, E. and Jones D. “The Genus Brochothrix”. ‘’The Prokaryotes’’. 2006. p. 447-491.
[25] Vangelova, L. “Botulinum Toxin: A Poison That Can Heal”. FDA Consumer Magazine. 1995.
[30] "Spoiled Meat". Spoiled Meat Niche Group's Image. 2008.
Edited by Steven Lee, Jade Nguyen, Sarah Paek, June Tse, Amy Vo, students of Rachel Larsen