Opportunistic Infections Caused by Serratia marcescens: Difference between revisions
No edit summary |
|||
| (106 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Curated}} | |||
==Introduction== | ==Introduction== | ||
[[Image:Serratia_marcescens.jpg|thumb|300px|right|Red colony of <br><i>Serratia marcescens</i>. Image from:http://www.retroscope.eu/wordpress/serratia-marcescens/serratiamarcescenscolony/]] | |||
<br>By [Brittany Currey]<br> | <br>By [Brittany Currey]<br> | ||
<br> | <br> | ||
<i>Serratia marcescens</i>, a rod-shaped gram-negative bacterium (0.5-0.8 µm in diameter and 0.9-2.0 µm in length), is a member of the Enterobacteriaceae family (4, 11). It is a facultative anaerobe that can grow in the presence and absence of oxygen at temperatures between 30°C and 37°C. It metabolizes by either respiratory or fermentative processes classifying it as a chemoorganotroph (4). Ubiquitous in nature, <i>S. marcescens</i> is found on dead organic material, while some inhabit soil, water, air, plants, animals, or food. In particular, food sources that provide a nutrient rich environment include starchy variants (11). The bacterium propels itself in these different environments using peritrichous flagellum (4). | |||
Identifiable in ecological niches by their red pigment, prodigiosin (2-methyl-3-penty-6-methoxyprodigiosin), S marcescens can resemble blood ( | Identifiable in ecological niches by their red pigment, prodigiosin (2-methyl-3-penty-6-methoxyprodigiosin), <i>S. marcescens</i> can resemble blood (11). Since this pigment is easily recognized in certain strains due to its red coloring, it has been extensively used as a biological marker (31). However, not all strains contain red pigment and most clinical isolates of <i>S. marcescens</i> have to rely on other biological markers since the majority of bacteria are not pigmented (10). Also, prodigiosin has antibacterial, antifungal, antiprotozoan, and immunosuppressant activity. Environmental signals such as temperature, phosphate limitation, and medium components regulate prodigiosin production. Prodigiosin is not the only secreted product, many more include protease, nucleases, lipase, chitinase, the biosurfactant serrawettin, and hemolysin (29). | ||
Although | Although <i>S. marcescens</i> was considered to be an innocuous, non-pathogenic organism, over the last two decades they have become an opportunist pathogen causing nosocomial infections (11). A broad range of hospital-acquired infections caused by <i>S. marcescens</i> include respiratory tract infections, urinary tract infections (UTI), septicaemia, meningitis, pneumonia, conjunctivitis wound and eye infections, osteomyelitis, keratoconjunctivitis, keratitis, endophthalmitis and endocarditis (5, 11, 14, 15, 28). Reports have shown rare cases of <i>S. marcescens</i> in nonhospital settings. These cases are linked to patients with immune deficiencies or chronic debilitating diseases. <i>S. marcescens</i> are also capable of causing diseases in a diverse group of organisms including animals, coral, insects, and plants (8, 11). | ||
Many problems arise in treating nosocomial infections because of resistance to a variety of antibiotics, such as cephalosporins, aztreonam, imipenem, cefotaxime, and ceftazidime (16, 25). Therefore, novel treatment techniques are in need to eliminate infections without overuse of the antibiotic (29). | Many problems arise in treating nosocomial infections because of resistance to a variety of antibiotics, such as cephalosporins, aztreonam, imipenem, cefotaxime, and ceftazidime (16, 25). Therefore, novel treatment techniques are in need to eliminate infections without overuse of the antibiotic (29). | ||
==Hydrophobicity== | |||
[[Image:Serratia_marcescens_biofilm.jpg|thumb|400px|left|Figure 1: A-F) Biofilm development of <br><i>Serratia marcescens</i> MG1 supplemented with glucose over a 10-day period. Image from:http://jb.asm.org/cgi/reprint/189/1/119]] | |||
<br><i>S. marcescens</i> are pathogenic and virulent because of their ability to adhere to host epithelial surfaces. Studies have shown that <i>S. marcescens</i> are capable of adhering to hydrocarbons and polystyrene allowing them to infect hosts by cell-surface hydrophobicity (2). Not only do these organisms adhere to biotic substrates but they are also found on abiotic substrates such as contact lenses. These organisms may possess pili that foster their adherence as well as an O-antigen that has a strong influence on the adhesion to abiotic and biotic surfaces (11). In particular, such interactions are mediated by large surface pili called type 1 fimbriae (13). | |||
<i>S. marcescens</i> possessing type 1 fimbriae use biofilms to regulate quorum sensing (12). Biofilms are formed when <i>S. marcescens</i> aggregate together and attach to a surface (Figure 1). When these microbes aggregate together they can communicate with one another via quorum sensing. Organisms can benefit from biofilms because they are proposed to provide protection against external stress. However, when cells reach high densities within a biofilm this may result in stressful environments classified by limited nutrients and oxygen, nonoptimal pH, and accumulation of metabolic by-products. Therefore, it is important that <i>S. marcescens</i> can evolve and adapt to different microniches within biofilms (15). Biofilms play an important role in disease of humans and plants, and Koh <i>et al.</i> (2007) have characterized the development of the microcolony-type biofilm of <i>S. marcescens</i> MG1. <i>S. marcescens</i> showed a strong, diversifying selection occurring within the biofilm to enhance the adaptive potential of the microbial population. Moreover, different stages of biofilm development showed specialized traits displaying different phenotypic variants (Figure 1). Studies will better understand microbial evolution processes by functional diversification within the biofilm (15). Ultimately, this information could be useful to better understand the diversification of <i>S. marcescens</i> strains impacting humans and plants. <br> | |||
< | |||
==Antibiotic Susceptibility== | ==Antibiotic Susceptibility== | ||
<br>The external face of the outer membrane of S. marcescens is formed by lipopolysaccharide (LPS) which is responsible for the biological activity of an endotoxin in its ability to cause disease. The outer membrane that surrounds this gram negative bacterium cell protects it from toxic agents by slowing their penetration and hindering their access to the target | <br>The external face of the outer membrane of <i>S. marcescens</i> is formed by lipopolysaccharide (LPS) which is responsible for the biological activity of an endotoxin in its ability to cause disease. The outer membrane that surrounds this gram negative bacterium cell protects it from toxic agents by slowing their penetration and hindering their access to the target site. LPS is composed of parts that include the O-antigen, lipid A, and the core (11). | ||
<i>S. marcescens</i> can become resistant to pencillins via two methods. First, <i>S. marcescens</i> has been shown to decrease its outer-membrane permeability and second, this bacterium uses beta lactamase to cleave the beta lactam ring of pencillin which inhibits the entry of the antibiotic (11). | |||
In several Enterobacteriaceae species, efflux by RND-type transporters is known to confer resistance to an antibiotic, tigecycline. Hornsey et al. (2010) examined tigecycline in | In several Enterobacteriaceae species, efflux by RND-type transporters is known to confer resistance to an antibiotic, tigecycline. Hornsey <i>et al.</i> (2010) examined tigecycline in <i>S. marcescens</i> clinical isolates and laboratory-selected mutants to investigate efflux by RND-type transporters. In gram negative bacteria, RND-type efflux systems usually have a cytoplasmic membrane pump, a periplasmic component, and an outer membrane channel. However, the outer membrane channels in <i>S. marcescens</i> are not fully understood. In this present study, up-regulation of endogenous SdeXY-HasF mediated efflux is associated with tigecycline resistance in <i>S. marcescens</i> (12). | ||
Kumar and Worobee (2002) studied fluoroquinolone resistance of S. marcescens. They examined four fluoroquinolones (ofloxacin, ciprofloxacin, norfloxacin, and nalidixic acid) to determine the drug specificity of efflux. Western immunoblot experiments demonstrated the presence of at least two AcrA-like proteins in S. marcescens that resembled the periplasmic component of the pump. Thus, S. marcescens can become resistant to fluoroquinolones possibly by proton dependent efflux via RND pumps (16).<br> | Kumar and Worobee (2002) studied fluoroquinolone resistance of <i>S. marcescens</i>. They examined four fluoroquinolones (ofloxacin, ciprofloxacin, norfloxacin, and nalidixic acid) to determine the drug specificity of efflux. Western immunoblot experiments demonstrated the presence of at least two AcrA-like proteins in <i>S. marcescens</i> that resembled the periplasmic component of the pump. Thus, <i>S. marcescens</i> can become resistant to fluoroquinolones possibly by proton dependent efflux via RND pumps (16).<br> | ||
==Infection, Diagnosis, Prevention, and Treatment== | |||
<br> | |||
<i>S. marcescens</i> infections are known to be transmitted through hand-to-hand contact by medical personnel. In this case, solutions used for medical purposes, catheterizations, and needle punctures can be contaminated and infect patients(31). Patients may also be infected with <i>S. marcescens</i> because this bacterium is known to survive and grow well on disinfectants, antiseptics, and in distilled water. In addition, S. marcescens have been found in de-ionized water isolated from blood bags (11). | |||
Many cases of <i>S. marcescens</i> have been diagnosed by taking a biopsy of infected tissue, mucous, or blood. These samples were then grown in culture to see if a colony of <i>S. marcescens</i> appeared. | |||
After identification of <i>S. marcescens</i> infection control measures were used to prevent the spread of <i>S. marcescens</i>. Hospitals isolated patients, enforced hand disinfection along with glove and gown use, and investigated correct use of antimicrobial substances that would eliminate <i>S. marcescens</i> (39,40). Reports explain how contaminated breast milk by <i>S. marcescens</i> may be prevented with proper handling, preparation, and storage in NICUs (39). Furthermore, effective measures of environmental cleaning depends highly on the availability of trained service personnel. Therefore, additional personnel should be recruited to help disinfect environmental hand contact surfaces and multiuse equipment (39). | |||
< | |||
Reports have shown that if an outbreak of <i>S. marcescens</i> persists, some hospitals decided to close affected units to new admissions. Some closed beds to gain more space for infected patients. Although this method may prevent the spread of <i>S. marcescens</i>, a loss of income may result from a substantial decrease in patient numbers.(39) | |||
Once the patient is diagnosed with <i>S. marcescens</i>, doctors have to choose the right antibiotic treatment to eliminate <i>S. marcescens</i>. Many strains of this bacterium have been found and special care needs to be taken to treat patients without overuse of the drug. Overuse of the drug allows the bacterium to become resistant. Trained personnel should expose cultured biospies of <i>S. marcescens</i> taken from patients to different antibiotics to see which antibiotics are more effective at inhibiting growth of <i>S. marcescens</i>. <br> | |||
< | |||
==Cases in Humans== | ==Cases in Humans== | ||
[[Image:Augmentation_Rhinoplasty.jpg|thumb| | [[Image:Augmentation_Rhinoplasty.jpg|thumb|200px|right|Figure 2:A 0.5-cm protruding crusted papule on the tip on the nose infected with <i>Serratia marcescens</i>. Image from:http://onlinelibrary.wiley.com/doi/10.1111/j.1524-4725.2010.01788.x/pdf]] | ||
[[Image:Leg_ulcers.jpg|thumb|200px| | [[Image:Leg_ulcers.jpg|thumb|200px|left|Figure 3: | ||
Top: Right lower leg with multiple, sharply demarked ulcers and absceding nodules. | Top: Right lower leg with multiple, sharply demarked ulcers and absceding nodules. | ||
Middle:Fluctuating abscess on the malleolus lateralis. | Middle:Fluctuating abscess on the malleolus lateralis. | ||
Bottom:Rapidly improved after adapted therapy.Image from:http://www.john-libbey-eurotext.fr/e-docs/00/04/43/20/vers_alt/VersionPDF.pdf]] | Bottom:Rapidly improved after adapted therapy.Image from:http://www.john-libbey-eurotext.fr/e-docs/00/04/43/20/vers_alt/VersionPDF.pdf]] | ||
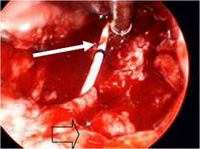
[[Image:Cervical.jpg|thumb|200px|right|Figure 4:Operative photograph of necrotic oropharynx | |||
from initial debridement at tertiary hospital. A nasogastric feeding tube traverses the wound (solid arrow), and | |||
the suction is resting on the prevertebral fascia. Empty | |||
arrow delineates remnant of soft palate.Image from: http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T7V-4V402DV-1-N&_cdi=5068&_user=7774802&_pii=S0165587608005053&_origin=gateway&_coverDate=03%2F31%2F2009&_sk=999269996&view=c&wchp=dGLbVzW-zSkWA&md5=88a67741405d6a92cc67e9cad7af5abf&ie=/sdarticle.pdf]] | |||
<br> | <br> | ||
First cases of nosocomial infections were recognized in humans in 1951 (31). | First cases of nosocomial infections were recognized in humans in 1951 (31). | ||
Dong Jin Ryu et al. (2010) presented a 26-year old woman who received augmentation rhinoplasty with a Gore-Tex implant. Twelve months following the initial plastic surgery, a second surgery was performed to fix the extrusion of the original implant with a silicone and cartilage implant. Two months later a folliculitis-like lesion formed on the tip of her nose. Signs of infection occurred when the lesion became tender and swelled enough to produce discharge. The lesion contained S. marcescens which was detected from a culture (Figure | Dong Jin Ryu <i>et al.</i> (2010) presented a 26-year old woman who received augmentation rhinoplasty with a Gore-Tex implant. Twelve months following the initial plastic surgery, a second surgery was performed to fix the extrusion of the original implant with a silicone and cartilage implant. Two months later a folliculitis-like lesion formed on the tip of her nose. Signs of infection occurred when the lesion became tender and swelled enough to produce discharge. The lesion contained <i>S. marcescens</i> which was detected from a culture (Figure 2). The lesion was treated with ceftazidime and ciprofloxacin, two antibiotics that produced significant effects on <i>S. marcescens</i>. The implant was later removed and the skin lesion healed three weeks later. | ||
Other Cutaneous infections caused by S. marcescens are uncommon but may be predisposed by immunocompromised conditions or pre-damaged skin (18). Langrock et al. (2008) presented a case in which a 73-year-old man contained multiple ulcers and painful nodules on the lower right leg as well as abscesses on the right malleolous lateralis. Skin cultures taken from the lesions were highly positive for S. marcescens. | Other Cutaneous infections caused by <i>S. marcescens</i> are uncommon but may be predisposed by immunocompromised conditions or pre-damaged skin (18). Langrock <i>et al.</i> (2008) presented a case in which a 73-year-old man contained multiple ulcers and painful nodules on the lower right leg as well as abscesses on the right malleolous lateralis. Skin cultures taken from the lesions were highly positive for <i>S. marcescens</i>. Oral antibiotic treatment with Pencillin was not effective at eliminating <i>S. marcescens</i>. Instead, topical therapy, repeated incision and draining of the abscesses, as well as the continuation of intravenous ertapenem improved skin lesions. The lesions improved gradually after 10 days of treatment and infection ceased completely, leaving hyperpigmented scars on the lower right leg (Figure 3). | ||
Five cases have been reported of Necrotizing fasciitis (NF) which infects soft tissues that spreads along fascia lines. Four of the five cases had lower extremity NF while one case involved an immunocompetent 2-year-old-who developed cervical NF infecting fascia planes of the head and neck (37) (Figure | Five cases have been reported of Necrotizing fasciitis (NF) which infects soft tissues that spreads along fascia lines. Four of the five cases had lower extremity NF while one case involved an immunocompetent 2-year-old-who developed cervical NF infecting fascia planes of the head and neck (37) (Figure 4). This patient with cervical NF was treated with serial debridements with irrigation of 3% hydrogen peroxide that was effective at removing necrotic tissue without need for external incisions. Unfornately, this patient was in an intensive care unit for a prolonged period of time due to sedation to tolerate wound packing and rapidly succumbed to the infection (37). | ||
Several outbreaks of | Several outbreaks of <i>S. marcescens</i> have been found in NICUs and PICUs throughout the world. Voelz <i>et al.</i> (2010) reported 27 studies in which 34 outbreaks of <i>S. marcescens</i> resulted. The Department of Hospital Epidemiology and Infection Control at the Johns Hopkins Hospital (JHH) in Baltimore, Maryland, detected an outbreak of multidrug–resistant (MDR) <i>S. marcescens</i> in a 36-bed NICU (26). The investigation took place from October 2004 through February 2005 in which one-third of the neonates hospitalized became infected or colonized the MDR bacterium. Many risk factors were assessed to determine the baseline rate of <i>S. marcescens</i> infection in the NICU prior to the outbreak. From medical records, they reviewed gestational age, low birth rate, mode of delivery (vaginal vs cesarean), location of delivery, (born at JHH vs born at another hospital and then transferred to JHH), low apgar score, previous surgery, previous mechanical ventilation, central venous catheterization, arterial catheterization, and inhalation medication therapy to name a few. The organism was found to grow in the endotracheal tubes of eight patients. MDR <i>S. marcescens</i> was detected in seven patients' blood, conjunctiva, peritoneal fluid, and/or periumbilical abscess fluid. Transmission of MDR <i>S. marcescens</i> was thought to be acquired by the location within the NICU. Reports show that the majority of the patients in pod 1 had acquired the organism, implying that there may have been an undetected environmental source or vector of transmission. The acquisition of the infection could also have been due to overcrowding of pod 1 where sufficient space was not provided between beds and proper hand hygiene was not maintained (26). Overall, Maragakis <i>et al.</i> (2008) believe that the organism was spread by transient carriage on the hands of healthcare personnel or on contaminated endotracheal respiratory care equipment. | ||
In Vienna, Austria, another outbreak of S. marcescens was detected in a NICU between October 1, 2000, and February 28, 2001. After eight patients were found to be colonized with the bacterium, the NICU was closed on December 27 for 10 days; to implement infection control measures and thoroughly disinfect the care unit. The primary case had septicemia with S. marcescens and after analysis of risk factors this neonate was suspected to have acquired the bacterium from his mother, who was treated 9 days with cefuroxime as a prophylactic treatment to avoid chorioamnionitis (inflammation of fetus) and intrauterine infection of the neonate after premature rupture of the membrane (1). A second case located next to the primary case also was infected with S. marcescens | In Vienna, Austria, another outbreak of <i>S. marcescens</i> was detected in a NICU between October 1, 2000, and February 28, 2001. After eight patients were found to be colonized with the bacterium, the NICU was closed on December 27 for 10 days; to implement infection control measures and thoroughly disinfect the care unit. The primary case had septicemia with <i>S. marcescens</i> and after analysis of risk factors this neonate was suspected to have acquired the bacterium from his mother, who was treated 9 days with cefuroxime as a prophylactic treatment to avoid chorioamnionitis (inflammation of fetus) and intrauterine infection of the neonate after premature rupture of the membrane (1). A second case located next to the primary case also was infected with <i>S. marcescens</i> conjunctivitis. All strains of <i>S. marcescens</i> were biologically identical, containing no pigmentation and were sensitive to imipenem S, cefotaxin R, cefpirom S, gentamicin S, amikacin S, trimethoprim S, and ciprofloxacin S. However, two cases were identified after the unit was closed and differed from the previous <i>S. marcescens</i> strains due to their red pigmentation and susceptibility of all antibiotics. To summarize, healthcare workers may have spread the bacterium after handling infected neonates and mothers may have been exposed to antibiotics that caused overgrowth of resistant organisms. This rapidly spreading infection was halted at an early stage with aggressive infection control measures(1). | ||
<br> | <br> | ||
==Cases in Animals== | ==Cases in Animals== | ||
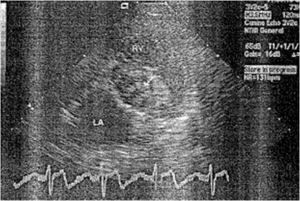
[[Image:Dog_1.jpg|thumb|300px|right|Figure 5: Endocardiogram. LA, left atrium; RV, right ventricle; RVOT, right ventricular outflow tract. The vegetations (arrow) are associated with the aortic valve. Image from:http://kenyon.illiad.oclc.org/illiad/illiad.dll?SessionID=A110039508U&Action=10&Form=75&Value=6412]] | |||
<br>Perez <i>et al.</i> (2011) reports nonhospital-acquired <i>S. marcescens</i> bacteria in a 12 year old male Dalmatian dog. The owners observed symptoms of a head tilt to the right, falling to the left, and nystagmus (involuntary eye movements). The primary veterinarian diagnosed the dog with canine idiopathic vestibular disease which affects neurological pathways. Five days later, the vestibular signs continued in the untreated dog along with vomiting. The dog was re-evaluated for a heart murmur and fever and referred to the Veterinary Teaching Hospital, College of Veterinary Medicine at North Carolina State University for additional diagnostic evaluation. An echocardiography found lesions on the aortic valve causing abnormal growth which was diagnosed as aortic valve endocarditis (Figure 5). Blood samples collected from different anatomical sites resulted in negative detection of <i>Anaplasma spp.</i>, <i>Borrelia burgdorferi</i>, <i>Ehrlichia canis</i> antibodies, and <i>Diofilaria immitis</i> antigen. Subsequently, the dog was treated with amplicillin, sulbactam, and doxycycline antibiotics. In conjunction, famotidine and metoclopramide were administerd for the vomiting. Despite aggressive therapy for congestive heart failure, the dog failed to respond to antimicrobials, developed opisthotonus (body frame forms a reverse bow due to severe spasms and hyperextension), and died within six hours after admission. Necropsy confirmed aortic valve endocarditis and were consistent with heart failure and septicemia. The blood samples returned in less than 12 hours, yielding a heavy growth of a pure culture of <i>S. marcescens</i>. This bacterium was resistant to the treatment antibiotics, however; it was susceptible to quinolones, third generation cephalosporins, imipenem, and aminoglycosides. This case of <i>S. marcescens</i> accumulated rapidly within the dog who was not hospitalized prior to infection. Thus, the dog acquired this infection in a community-related setting (31). | |||
A study from South Africa isolated 22 IV catheters from 100 dogs with parvoviral enteritis. Only 2 catheters tested positive for S marcescens (22, 31). In another case, a Doberman Pinscher underwent a tooth extraction and later developed fatal necrotizing fasciitis in association with S. marcescens. It was suggested that the bacteria was transmitted in the oral surgery by skin contamination at injection sites (31, 33). | Many other animal related cases have been described in hospital practices. The first documentation of nosocomial <i>Serratia spp</i> septicemia was reported in six dogs in 1973. Prior to <i>Serratia spp</i> septicemia diagnosis, all six dogs were previously diagnosed with debilitating diseases and later developed a fever under intensive hospital care with an indwelling jugular catheter (31, 41). Although the source of infection was not determined in these six cases, subsequent studies found that the high prevalence of <i>S. marcescens</i> in hospital related cases were due to contaminated benzalkonium chloride sponge pots used as an antiseptic. | ||
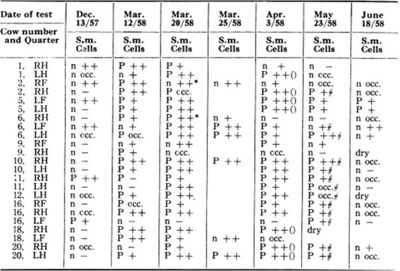
[[Image:cows.jpg|thumb|400px|left|Table 1: The record of isolation of <i>S. marcescens</i>. S.m. <i>S. marcescens</i>, P positive, n negative. Cells: - less than 500,000/ml, + 0.5-2 million, and ++ over 2 million. Image from:http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1614667/?page=2]] | |||
A study from South Africa isolated 22 IV catheters from 100 dogs with parvoviral enteritis. Only 2 catheters tested positive for <i>S. marcescens</i> (22, 31). In another case, a Doberman Pinscher underwent a tooth extraction and later developed fatal necrotizing fasciitis in association with <i>S. marcescens</i>. It was suggested that the bacteria was transmitted in the oral surgery by skin contamination at injection sites (31, 33). | |||
During the winter of 1952, a herd of twenty-four milking cattle were housed in a conventional type barn, where a mild outbreak of mastitis due to haemolytic <i>Staphylococcus aureus</i> occurred. In this community setting, they also isolated <i>S. marcescens</i> from incubated milk (Table 1). The cows’ symptoms included swelling of the gland and visibly abnormal milk clots. The organism was found to be susceptible to four or five grams of neomycin and was eliminated over a three day period (Table 1)(3). | |||
<br> | <br> | ||
==Research== | |||
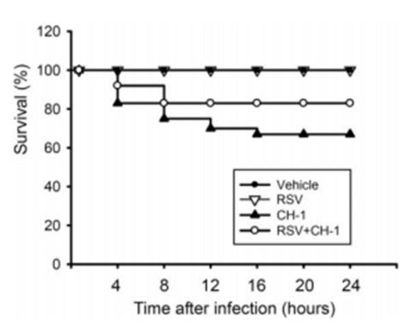
[[Image:RSV.jpg|thumb|400px|left|Figure 6: Survival rate of rats infected with <i>S. marcescens</i>, with or without RSV pretreatment. Rats were pretreated with RSV or saline for 3 days and then | |||
inoculated with or without <i>S. marcescens</i>. Image from: http://www.jleukbio.org/content/83/4/1028.full.pdf+html]] | |||
<br>Many treatment methods have been limited by the emergence of multiple drug-resistant strains of <i>S. marcescens</i>. In one study, resveratrol (RSV; trans-3,5,4’-trihydroxystilbene) was studied to see if it has in vivo prophylactic or therapeutic potential in which it could possibly prevent and treat <i>S. marcescens</i> infections. RSV has many diverse biological effects including anti-cancer, anti-inflammation, anti-diabetics, and cancer chemoprevention. Chia-Chen Lu <i>et al.</i> (2008) evaluated the effects of RSV against pneumonia which is an infection characterized by cough with sputum production, high fever, and mortality of different degrees. The body initiates a response to the build-up of infectious particles by using alveolar macrophages (AV) to synthesize and secrete a wide array of pro-inflammatory proteins (cytokines and chemokines) in the cell. Rats were used as a model system to study acute pneumonia induced by instillation of <i>S. marcescens</i>. Figure 6 shows that the survival rate of animals was 67% without RSV pretreatment but was 83% for animals pretreated with RSV. Results showed that RSV pretreatment for 3 days increased AM infiltration, elevated NK cell activity, decreased bacterial load in lungs, and overall decreased mortality rate. Therefore, these results suggest that RVS might be beneficial as a preventative measure against patients with <i>S. marcescens</i>-induced acute pneumonia (23). | |||
[[Image:chitosan.jpg|thumb|400px|right|Figure 7: Effect of chitosan concentration on the antibacterial activity of strain ZJ-S0801 of <i>S. marcescens</i>. Columns with the same letters are not significantly different. | |||
Image from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1517-83822011000100013]] | |||
Li <i>et al.</i> (2011) studied <i>S. marcescens</i> isolated from an edible cactus plant and silkworms to evaluate the effects of the bactericidal activity of chitosan. Chitosan is industrially obtained by N-deactylation of shrimp and crapshell chitin. Previously, chitosan has shown a potential to control bacterial septicemic disease of silkworms caused by <i>S. marcescens</i> (19, 20). Along with chitosan being useful as a pharmaceutical agent in biomedicine, it is also useful as an antimicrobial compound in agriculture, a potential elicitor of plant defense responses, an additive in the food industry, and a hydrating agent in cosmetics. Chitosan exhibits antimicrobial activity against fungi, algae, and some bacteria. The antimicrobial uses of chitosan need to be further studied in depth because differences in its derivatives are affected by the degree of deacetylation, the molecular weight, the ionic strength, the pH, and the temperature (9). Li <i>et al.</i> (2011) conducted further research to examine the activity of chitosan against plant species affected with <i>S. marcescens</i>. The surviving cell numbers of the <i>S. marcescens</i> strain ZJ-S0801 decreased with increasing amounts of chitosan (Figure 7). Strong antimicrobial activity of chitosan was shown against plant associated <i>S. marcescens</i>. This research of chitosan will be useful in the control of contaminated fruit and vegetable products since the infected plant strain may play a possible role in human and animal food sources(19).<br> | |||
==Conclusion== | ==Conclusion== | ||
<br> | <br><i>S. marcescens</i> is an opportunistic pathogen causing a plethora of nosocomial infections in humans and some cases have been reported in animals. Many strains have become resistant to a variety of drugs. Future research, should evaluate different measures to prevent outbreaks of <i>Serratia marcescens</i> by evaluating antibiotics and the mode of entry into the bacterium. Pathogenic pathways should be more fully defined to understand how resistant <i>S. marcescens</i> infections may become vulnerable to different treatment methods. <br> | ||
==References== | ==References== | ||
<br>[http://www.jstor.org/stable/30144703]Assadian, Ojan, Berger, Angelika, Aspöck, Christoph, Mustafa, Stefan, Kohlauser, Christina, Hirschl, Alexander. 2002. Nosocomial Outbreak of <i>Serratia marcescens</i> in a Neonatal Intensive Care Unit. Infection control and Hospital Epidemiology 23: 457-463<br> | |||
<br>[http://mic.sgmjournals.org/cgi/content/abstract/135/8/2277]Bar-Ness, Ronit, and Rosenberg, Mel. 1989. Putative Role of 70 kDa Outer-surface Protein in Promoting Cell-surface Hydrophobicity of <i>Serratia marcescens</i>RZ. General Microbiology 135: 2277-2281<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1614667/]Barnum, D.A., Thackeray, E.L., and Fish, N.A. 1958. An Outbreak of Mastitis Caused by <i>Serratia marcescens</i>. Canadian Journal of Comparative Medicine 22: 392-395<br> | |||
<br>[http://books.google.com/books?id=yviRIumE5HgC&pg=PA226&lpg=PA226&dq=Breed,+R.S.,+Murray,+E.G.D.,+and+Smith,+N.R.+1957.Bergey%E2%80%99s+Manual+of+Determinative+Bacteriology.&source=bl&ots=pk4zAxYPP_&sig=nKo7UsiEJc1YvY6mQZJNYCmt5e0&hl=en&ei=YTu2TYfAO6ni0QH2n73RDw&sa=X&oi=book_result&ct=result&resnum=2&ved=0CCUQ6AEwAQ#v=onepage&q=Breed%2C%20R.S.%2C%20Murray%2C%20E.G.D.%2C%20and%20Smith%2C%20N.R.%201957.Bergey%E2%80%99s%20Manual%20of%20Determinative%20Bacteriology.&f=false]Breed, R.S., Murray, E.G.D., and Smith, N.R. 1957.Bergey’s Manual of Determinative Bacteriology. 9th edition. Page 187<br> | |||
<br>[http://jb.asm.org/cgi/content/full/190/1/213]Castelli, Maria E., Fedrigo, Griselda V., Clementín, Ana L., Ielmini, M. Verónica, Feldman, Mario F., and Véscovi, Eleonora G. 2008. Enterobacterial Common Antigen Integrity Is a Checkpoint for Flagellar Biogenesis in <i>Serratia marcescens</i>. Bacteriology 190: 213-220<br> | |||
<br>[http://jac.oxfordjournals.org/content/52/2/176.full.pdf]Chen, Jing, Kuroda, Teruo, Huda, Nazmul, Mizushima, Tohru, and Tsuchiya, Tomofusa. 2003. An RND-type multidrug efflux pump SdeXY from <i>Serratia marcescens</i>. Antimicrobial Chemotherapy 52: 176-179<br> | |||
<br>[http://jama.ama-assn.org/content/197/13/1059.full.pdf]Clayton, Edwin, and Graevenitz, Alexander V. 1966. Nonpigmented <i>Serratia marcescens</i>. American Medical Association 197: 111-116<br> | |||
<br>[http://mic.sgmjournals.org/cgi/reprint/152/7/1899.pdf]Coulthurst, Sarah J., Williamson, Neil R., Harris, Abigail K.P., Spring, David R., and Salmond, George P.C. 2006. Metabolic and regulatory engineering of <i>Serratia marcescens</i>: mimicking phage-mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology 152: 1899-1911<br> | |||
<br>[http://jb.asm.org/cgi/content/short/189/21/7653]Fineran, Peter C., Williamson, Neil R., Lilley, Kathryn S., and Salmond, George P.C. 2007. Virulence and Prodigiosin Antibiotic Biosynthesis in <i>Serratia</i> Are Regulated Pleiotropically by the GGDEF/EAL Domain Protein, PigX. Bacteriology 189: 7653-7662<br> | |||
<br>[http://jb.asm.org/cgi/content/abstract/190/22/7453]Haddix, Pryce L., Jones, Sarah, Patel, Pratik, Burnham, Sarah, Knights, Kaori, Powell, Joan N. and LaForm, Amber. 2008. Kinetic Analysis of Growth Rate, ATP, and Pigmentation Suggests an Energy-Spilling Function for the Prodigiosin of <i>Serratia marcescens</i>. Bacteriology 190: 7453-7463<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/9368530]Hejazi, A. and Falkiner, F.R. 1997. <i>Serratia marcescens</i>. Medical Microbiology 46: 903-912<br> | |||
<br>[http://jac.oxfordjournals.org/content/65/3/479.abstract]Hornsey, Michael, Ellington, Matthew J., Doumith, Michel, Hudson, Sue, Livermore, David M., and Woodford, Neil. 2010. Tigecycline resistance in <i>Serratia marcescens</i> associated with up-regulation of the SdeXY-HasF efflux system also active against ciprofloxacin and cefpirome. Antimicrobial Chemotherapy 65: 479-482<br> | |||
<br>[http://aem.asm.org/cgi/content/short/74/11/3461]Kalivoda, Eric J., Stella, Nicholas A., O’Dee, Dawn M., Nau, Gerard J., and Shanks, Robert M.Q. 2008. The Cyclic AMP-Dependent Catabolite Repression System of <i>Serratia marcescens</i> Mediates Biofilm Formation through Regulation of Type 1 Fimbriae. Applied and Environmental Microbiology 74: 3461-3470<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/17043106]Kida, Yutaka, Inoue, Hiroyoshi, Shimizu, Takashi, and Kuwano, Koichi. 2007. <i>Serratia marcescens</i> Serralysin Induces Inflammatory Responses through Protease-Activated Receptor 2. Infection and Immunity 75: 164-174<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1797207/]Koh, Kai S., Lam, Kin W., Alhede, Morten, Queck, Shu Y., Labbate, Maurizio, Kjelleberg, Staffan, and Rice, Scott A. 2007. Phenotypic Diversification and Adaptation of <i>Serratia marcescens</i> MG1 Biofilm-Derived Morphotypes. Bacteriology 189: 119-130<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/12356807]Kumar, Ayush, and Worobee, Elizabeth A. 2002. Fluoroquinolone resistance of <i>Serratia marcescens</i>: involvement of a proton gradient-dependent efflux pump. Antimicrobial Chemotherapy 50: 593-596<br> | |||
<br>[http://jb.asm.org/cgi/content/abstract/189/7/2702]Labbate, Maurizio, Zhu, Hua, Thung, Leena, Bandara, Rani, Larsen, Martin R., Willcox, Mark D.P., Givskov, Michael, Rice, Scott A., and Kjelleberg, Staffan. 2007. Quorum-Sensing Regulation of Adhesion in <i>Serratia marcescens</i> MG1 Is Suface Dependent. Bacteriology 189: 3461-3470<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/18955204]Langrock, Marie-Lousie, Linde, Hans-Jörg, Landthaler, Michael, and Karrer, Sigrid. 2008. Leg ulcers and abscesses caused by <i>Serratia marcescens</i>. Eur J Dermatol 18: 705-707<br> | |||
<br>[http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1517-83822011000100013]Li, Bin, Yu, Rongrong, Liu, Baoping, Tang, Qiaomei, Zhang, Guoqing, Wang, Yanli, Xie, Guanlin, Sun, Guochang. 2011. Characterization and comparison of <i>Serratia marcescens</i> isolated from edible cactus and from silkworm for virulence potential and chitosan susceptibility. Microbiology 42: 96-104<br> | |||
<br>[http://www.jstage.jst.go.jp/article/aez/45/1/45_145/_article]Li, B., Xu, L.H., Chen, X.L., Lui, B.P., Zhu, B., Fang, Y., Qui, W., and Xie, G.L. 2009. Effect of chitosan solution on the bacterial septicemia disease of Bombyx mori (Lepidoptera: Bombycidae) caused by <i>Serratia marcescens</i>. Appl. Entomol. Zool. 45: 145-152<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/21340420]Lima, KV, Carvalho,RG, Carneiro, IC, Lima, JL, Sousa Cde, O., Loureiro, EC, Sá, LL, and Bastos, FC. I 2011. Outbreak of neonatal infection by an endemic clone of <i>Serratia marcescens</i>. Revista da Sociedade Brasilleira de Medicina Tropical 44: 106-109<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/11991409]Lobetti RG, Joubert KE, Picard J, et al. 2002. Bacterial colonization of intravenous catheters in youngs dogs suspected to have parvoviral enteritis. Am Vet Med Assoc 220: 1321-1324<br> | |||
<br>[http://www.jleukbio.org/content/83/4/1028.abstract]Lu, Chia-Chen, Lai, Hsin-Chih, Hsieh, Shang-Chen, and Chen, Jan-Kan. 2008. Resveratrol ameliorates <i>Serratia marcescens</i> induced acute pneumonia in rats. Leukocyte Biology 83: 1028-1037<br> | |||
<br>[http://cat.inist.fr/?aModele=afficheN&cpsidt=19681953]Makimura, Yutaka, Asai, Yasuyuki, Sugiyama, Akiko, and Ogawa, Tomohiko. 2007. Chemical structure and imunobiological activity of lipid A from <i>Serratia marcescens</i> LPS. Medical Microbiology 56: 1440-1446<br> | |||
<br>[http://aac.asm.org/cgi/reprint/48/3/716]Mammeri, Hedi, Poirel, Laurent, Bemer, Pascal, Drugeon, Henri, and Nordmann, Patrice. 2004. Resistance to Cefepime and Cefpirome Due to a 4-Amino-Acid Deletion in the Chromosome-Encoded AmpC β-Lactamase of a <i>Serratia marcescens</i> Clinical Isolate. Antimicrobial Agents and Chemotherapy 48: 716-720<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/18419363?dopt=Abstract]Maragakis, Lisa L., Winkler, Amy, Tucker, Margaret G., Cosgrove, Sara E., Ross, Tracy, Lawson, Edward, Carroll, Karen C., Perl, Trish M. 2008. Outbreak of Multidrug-Resistant <i>Serratia marcescens</i> Infection in a Neonatal Intensive Care Unit. Infection Control and Hospital Epidemiology 29: 418-423<br> | |||
<br>[http://aac.asm.org/cgi/content/short/53/12/5230]Maseda, Hideaki, Hashida, Yumiko, Konaka, Rumi, Shirai, Akihiro, and Kourai, Hiroki. 2009. Mutational Upregulation of a Resistance-Nodulation-Cell Division-Type Multidrug Efflux Pump, SdeAB, upon Exposure to a Biocide, Cetylpyridinium Chloride, and Antibiotic Resistance in <i>Serratia marcescens</i>. Antimicrobial Agents and Chemotherapy 53: 5230-5235<br> | |||
<br>[http://jb.asm.org/cgi/content/short/190/2/648]Matsuo, Taira, Chen, Jing, Minato, Yusuke, Ogawa, Wakano, Mizushima, Tohru, Kuroda, Teruo, and Tscuhiya, Tomofusa. 2008. SmdAB, a Heterodimeric ABC-Type Multidrug Efflux Pump, in <i>Serratia marcescens</i>. Bacteriology 190: 648-654<br> | |||
<br>[http://aem.asm.org/cgi/content/short/73/20/6339]Morohoshi, Tomohiro, Shiono, Toshitaka, Takidouchi, Kiyomi, Kato, Masashi, Kato, Norihiro, Kato, Junichi, and Ikeda, Tsukasa. 2007. Inhibition of Quorum Sensing in <i>Serratia marcescens</i> AS-1 by Synthetic Analogs of N-Acylhomoserine Lactone. Applied and Environmental Microbiology 73: 6339-6344<br> | |||
<br>[http://www.theannals.com/cgi/reprint/41/6/1077]Nicasio, Anthony M., Quintiliani Jr, Richard, DeRyke, C Andrew, Kuti, Joseph L., Nicolau, David P. 2007. Treatment of <i>Serratia marcescens</i> Meningitis with Prolonged Infusion of Meropenem. The Annals of Pharmacotherapy 41: 1077-1081<br> | |||
<br>[http://www.jaaha.org/cgi/content/abstract/43/5/258]Perez, Cristina, Fujii, Yoko, Fauls, Megan, Hummel, James, and Breitschwerdt, Edward. 2011. Fatal Aortic Endocarditis Associated with Community-Acquired <i>Serratia marcescens</i> Infection in a Dog. American Animal Hospital Association 47: 133-137<br> | |||
<br>[http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2010.01901.x/full]Petty, Nicola K., Foulds, Ian J., Pradel, Elizabeth, Ewbank, Jonathan J., and Salmond George P.C. 2006. A generalized transducing phage (ϕIF3) for the genomically sequenced <i>Serratia marcescens</i> strain DB11: a tool for functional genomics of an opportunistic human pathogen. Microbiology 152: 1701-1708<br> | |||
<br>[http://www.vri.cz/docs/vetmed/53-11-629.pdf]Plavec T, Zdovc I, Juntes P, et al. 2002. Necrotizing fasciitis caused by <i>Serratia marcescens</i> after tooth extraction in a Doberman Pinscher: a case report. Veterinarni Medicina 53: 629-635<br> | |||
<br>[http://iai.asm.org/cgi/content/abstract/9/3/550]Quarles, John M., Belding, Ralph C., Beaman Cabrera, Teofila, and Gerhardt, Philipp. 1974. Hemodialysis Culture of <i>Serratia marcescens</i> in a Goat-Artificial Kidney-Fermentor System. Infection and Immunity 9: 550-558<br> | |||
<br>[http://pubs.acs.org/doi/pdf/10.1021/bm034130m]Rabea, Entsar I., Badaway, Mohamed E.T., Stevens, Christian V.m Smagghe, Guy,and Steurbaut, Walter. 2003. Chitosan as Antimicrobial agent: Applications and Mode of Action.Biomacromolecules 4: 1457-1465 <br> | |||
<br>[http://onlinelibrary.wiley.com/doi/10.1111/j.1524-4725.2010.01788.x/pdf]Ryu, Dong Jin, Oh, Sang Ho, Choi, Yoon Jin, and Lee, Ju Hee. 2010. A Case of <i>Serratia marcescens</i> After Augmentation Rhinoplasy. American Society for Dermatologic Surgery 36: 2079-2081<br> | |||
<br>[http://www.sciencedirect.com/science?_ob=MImg&_imagekey=B6T7V-4V402DV-1N&_cdi=5068&_user=7774802&_pii=S0165587608005053&_origin=gateway&_coverDate=03%2F31%2F2009&_sk=999269996&view=c&wchp=dGLbVzW-zSkWA&md5=88a67741405d6a92cc67e9cad7af5abf&ie=/sdarticle.pdf]Statham, Melissa M., Vohra, Amit, Mehta, Deepak K., Baker, Troy, Sarlay, Robert, and Rutter, Michael J. 2009. <br><i>Serratia marcescens</i> causing cervical necrotizing oropharyngitis. International Jouranl of Pediatric Otorhinolaryngology 73: 467-473<br> | |||
<br>[http://www.springerlink.com/content/y1k1t1jb3fpw09xe/]Trilla, A. 1994. Epidemiology of nosocomial infections in adult intensive care units. Intensive Care Med 20: S1-S4<br> | |||
<br>[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B7GVY-4X9V336-1&_user=7774802&_coverDate=03%2F31%2F2010&_rdoc=1&_fmt=high&_orig=gateway&_origin=gateway&_sort=d&_docanchor=&view=c&_searchStrId=1749203615&_rerunOrigin=google&_acct=C000062877&_version=1&_urlVersion=0&_userid=7774802&md5=7052ee8c840d63d9d48b6e59e61aaf0e&searchtype=a]Voelz, Alexander, Müller, Andreas, Gillen, Julia, Le, Celine, Dresbach, Till, Engelhart, Steffen, Exner, Martin, Bates, Christine J., and Simon, Arne. 2010. Outbreaks of <i>Serratia marcescens</i> in neonatal and pediatric intensive care units: Clinical aspects, risk factors and management. Int. J. Hyg. Environ. Health 213: 79-87<br> | |||
<br>[http://www.ncbi.nlm.nih.gov/pubmed/21153042]Vonberg, Weitzel-Kage, Behnke, and Gastmeier. 2011. Worldwide Outbreak Database: the largest collection of nosocomial outbreaks. Infection 39: 29-34<br> | |||
<br>[http://ukpmc.ac.uk/abstract/MED/4580258]Wilkins RJ. 1973. <i>Serratia marcescens</i> septicaemia in the dog. Small Anim Pract 14: 205-15<br> | |||
Edited by student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl10.html BIOL 238 Microbiology], 2011, [http://www.kenyon.edu/index.xml Kenyon College]. | Edited by [mailto:curreyb@kenyon.edu Brittany Currey],student of [mailto:slonczewski@kenyon.edu Joan Slonczewski] for [http://biology.kenyon.edu/courses/biol238/biol238syl10.html BIOL 238 Microbiology], 2011, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 14:32, 23 July 2011
Introduction
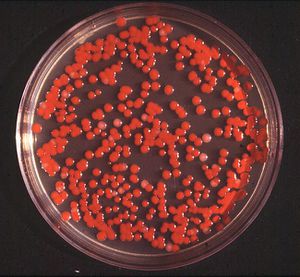
Serratia marcescens. Image from:http://www.retroscope.eu/wordpress/serratia-marcescens/serratiamarcescenscolony/
By [Brittany Currey]
Serratia marcescens, a rod-shaped gram-negative bacterium (0.5-0.8 µm in diameter and 0.9-2.0 µm in length), is a member of the Enterobacteriaceae family (4, 11). It is a facultative anaerobe that can grow in the presence and absence of oxygen at temperatures between 30°C and 37°C. It metabolizes by either respiratory or fermentative processes classifying it as a chemoorganotroph (4). Ubiquitous in nature, S. marcescens is found on dead organic material, while some inhabit soil, water, air, plants, animals, or food. In particular, food sources that provide a nutrient rich environment include starchy variants (11). The bacterium propels itself in these different environments using peritrichous flagellum (4).
Identifiable in ecological niches by their red pigment, prodigiosin (2-methyl-3-penty-6-methoxyprodigiosin), S. marcescens can resemble blood (11). Since this pigment is easily recognized in certain strains due to its red coloring, it has been extensively used as a biological marker (31). However, not all strains contain red pigment and most clinical isolates of S. marcescens have to rely on other biological markers since the majority of bacteria are not pigmented (10). Also, prodigiosin has antibacterial, antifungal, antiprotozoan, and immunosuppressant activity. Environmental signals such as temperature, phosphate limitation, and medium components regulate prodigiosin production. Prodigiosin is not the only secreted product, many more include protease, nucleases, lipase, chitinase, the biosurfactant serrawettin, and hemolysin (29).
Although S. marcescens was considered to be an innocuous, non-pathogenic organism, over the last two decades they have become an opportunist pathogen causing nosocomial infections (11). A broad range of hospital-acquired infections caused by S. marcescens include respiratory tract infections, urinary tract infections (UTI), septicaemia, meningitis, pneumonia, conjunctivitis wound and eye infections, osteomyelitis, keratoconjunctivitis, keratitis, endophthalmitis and endocarditis (5, 11, 14, 15, 28). Reports have shown rare cases of S. marcescens in nonhospital settings. These cases are linked to patients with immune deficiencies or chronic debilitating diseases. S. marcescens are also capable of causing diseases in a diverse group of organisms including animals, coral, insects, and plants (8, 11).
Many problems arise in treating nosocomial infections because of resistance to a variety of antibiotics, such as cephalosporins, aztreonam, imipenem, cefotaxime, and ceftazidime (16, 25). Therefore, novel treatment techniques are in need to eliminate infections without overuse of the antibiotic (29).
Hydrophobicity
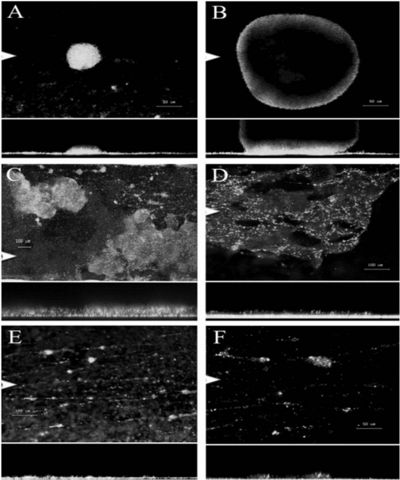
Serratia marcescens MG1 supplemented with glucose over a 10-day period. Image from:http://jb.asm.org/cgi/reprint/189/1/119
S. marcescens are pathogenic and virulent because of their ability to adhere to host epithelial surfaces. Studies have shown that S. marcescens are capable of adhering to hydrocarbons and polystyrene allowing them to infect hosts by cell-surface hydrophobicity (2). Not only do these organisms adhere to biotic substrates but they are also found on abiotic substrates such as contact lenses. These organisms may possess pili that foster their adherence as well as an O-antigen that has a strong influence on the adhesion to abiotic and biotic surfaces (11). In particular, such interactions are mediated by large surface pili called type 1 fimbriae (13).
S. marcescens possessing type 1 fimbriae use biofilms to regulate quorum sensing (12). Biofilms are formed when S. marcescens aggregate together and attach to a surface (Figure 1). When these microbes aggregate together they can communicate with one another via quorum sensing. Organisms can benefit from biofilms because they are proposed to provide protection against external stress. However, when cells reach high densities within a biofilm this may result in stressful environments classified by limited nutrients and oxygen, nonoptimal pH, and accumulation of metabolic by-products. Therefore, it is important that S. marcescens can evolve and adapt to different microniches within biofilms (15). Biofilms play an important role in disease of humans and plants, and Koh et al. (2007) have characterized the development of the microcolony-type biofilm of S. marcescens MG1. S. marcescens showed a strong, diversifying selection occurring within the biofilm to enhance the adaptive potential of the microbial population. Moreover, different stages of biofilm development showed specialized traits displaying different phenotypic variants (Figure 1). Studies will better understand microbial evolution processes by functional diversification within the biofilm (15). Ultimately, this information could be useful to better understand the diversification of S. marcescens strains impacting humans and plants.
Antibiotic Susceptibility
The external face of the outer membrane of S. marcescens is formed by lipopolysaccharide (LPS) which is responsible for the biological activity of an endotoxin in its ability to cause disease. The outer membrane that surrounds this gram negative bacterium cell protects it from toxic agents by slowing their penetration and hindering their access to the target site. LPS is composed of parts that include the O-antigen, lipid A, and the core (11).
S. marcescens can become resistant to pencillins via two methods. First, S. marcescens has been shown to decrease its outer-membrane permeability and second, this bacterium uses beta lactamase to cleave the beta lactam ring of pencillin which inhibits the entry of the antibiotic (11).
In several Enterobacteriaceae species, efflux by RND-type transporters is known to confer resistance to an antibiotic, tigecycline. Hornsey et al. (2010) examined tigecycline in S. marcescens clinical isolates and laboratory-selected mutants to investigate efflux by RND-type transporters. In gram negative bacteria, RND-type efflux systems usually have a cytoplasmic membrane pump, a periplasmic component, and an outer membrane channel. However, the outer membrane channels in S. marcescens are not fully understood. In this present study, up-regulation of endogenous SdeXY-HasF mediated efflux is associated with tigecycline resistance in S. marcescens (12).
Kumar and Worobee (2002) studied fluoroquinolone resistance of S. marcescens. They examined four fluoroquinolones (ofloxacin, ciprofloxacin, norfloxacin, and nalidixic acid) to determine the drug specificity of efflux. Western immunoblot experiments demonstrated the presence of at least two AcrA-like proteins in S. marcescens that resembled the periplasmic component of the pump. Thus, S. marcescens can become resistant to fluoroquinolones possibly by proton dependent efflux via RND pumps (16).
Infection, Diagnosis, Prevention, and Treatment
S. marcescens infections are known to be transmitted through hand-to-hand contact by medical personnel. In this case, solutions used for medical purposes, catheterizations, and needle punctures can be contaminated and infect patients(31). Patients may also be infected with S. marcescens because this bacterium is known to survive and grow well on disinfectants, antiseptics, and in distilled water. In addition, S. marcescens have been found in de-ionized water isolated from blood bags (11).
Many cases of S. marcescens have been diagnosed by taking a biopsy of infected tissue, mucous, or blood. These samples were then grown in culture to see if a colony of S. marcescens appeared.
After identification of S. marcescens infection control measures were used to prevent the spread of S. marcescens. Hospitals isolated patients, enforced hand disinfection along with glove and gown use, and investigated correct use of antimicrobial substances that would eliminate S. marcescens (39,40). Reports explain how contaminated breast milk by S. marcescens may be prevented with proper handling, preparation, and storage in NICUs (39). Furthermore, effective measures of environmental cleaning depends highly on the availability of trained service personnel. Therefore, additional personnel should be recruited to help disinfect environmental hand contact surfaces and multiuse equipment (39).
Reports have shown that if an outbreak of S. marcescens persists, some hospitals decided to close affected units to new admissions. Some closed beds to gain more space for infected patients. Although this method may prevent the spread of S. marcescens, a loss of income may result from a substantial decrease in patient numbers.(39)
Once the patient is diagnosed with S. marcescens, doctors have to choose the right antibiotic treatment to eliminate S. marcescens. Many strains of this bacterium have been found and special care needs to be taken to treat patients without overuse of the drug. Overuse of the drug allows the bacterium to become resistant. Trained personnel should expose cultured biospies of S. marcescens taken from patients to different antibiotics to see which antibiotics are more effective at inhibiting growth of S. marcescens.
Cases in Humans
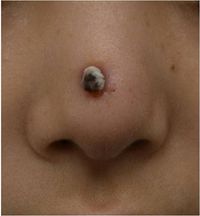
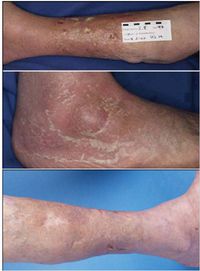
First cases of nosocomial infections were recognized in humans in 1951 (31).
Dong Jin Ryu et al. (2010) presented a 26-year old woman who received augmentation rhinoplasty with a Gore-Tex implant. Twelve months following the initial plastic surgery, a second surgery was performed to fix the extrusion of the original implant with a silicone and cartilage implant. Two months later a folliculitis-like lesion formed on the tip of her nose. Signs of infection occurred when the lesion became tender and swelled enough to produce discharge. The lesion contained S. marcescens which was detected from a culture (Figure 2). The lesion was treated with ceftazidime and ciprofloxacin, two antibiotics that produced significant effects on S. marcescens. The implant was later removed and the skin lesion healed three weeks later. Other Cutaneous infections caused by S. marcescens are uncommon but may be predisposed by immunocompromised conditions or pre-damaged skin (18). Langrock et al. (2008) presented a case in which a 73-year-old man contained multiple ulcers and painful nodules on the lower right leg as well as abscesses on the right malleolous lateralis. Skin cultures taken from the lesions were highly positive for S. marcescens. Oral antibiotic treatment with Pencillin was not effective at eliminating S. marcescens. Instead, topical therapy, repeated incision and draining of the abscesses, as well as the continuation of intravenous ertapenem improved skin lesions. The lesions improved gradually after 10 days of treatment and infection ceased completely, leaving hyperpigmented scars on the lower right leg (Figure 3).
Five cases have been reported of Necrotizing fasciitis (NF) which infects soft tissues that spreads along fascia lines. Four of the five cases had lower extremity NF while one case involved an immunocompetent 2-year-old-who developed cervical NF infecting fascia planes of the head and neck (37) (Figure 4). This patient with cervical NF was treated with serial debridements with irrigation of 3% hydrogen peroxide that was effective at removing necrotic tissue without need for external incisions. Unfornately, this patient was in an intensive care unit for a prolonged period of time due to sedation to tolerate wound packing and rapidly succumbed to the infection (37).
Several outbreaks of S. marcescens have been found in NICUs and PICUs throughout the world. Voelz et al. (2010) reported 27 studies in which 34 outbreaks of S. marcescens resulted. The Department of Hospital Epidemiology and Infection Control at the Johns Hopkins Hospital (JHH) in Baltimore, Maryland, detected an outbreak of multidrug–resistant (MDR) S. marcescens in a 36-bed NICU (26). The investigation took place from October 2004 through February 2005 in which one-third of the neonates hospitalized became infected or colonized the MDR bacterium. Many risk factors were assessed to determine the baseline rate of S. marcescens infection in the NICU prior to the outbreak. From medical records, they reviewed gestational age, low birth rate, mode of delivery (vaginal vs cesarean), location of delivery, (born at JHH vs born at another hospital and then transferred to JHH), low apgar score, previous surgery, previous mechanical ventilation, central venous catheterization, arterial catheterization, and inhalation medication therapy to name a few. The organism was found to grow in the endotracheal tubes of eight patients. MDR S. marcescens was detected in seven patients' blood, conjunctiva, peritoneal fluid, and/or periumbilical abscess fluid. Transmission of MDR S. marcescens was thought to be acquired by the location within the NICU. Reports show that the majority of the patients in pod 1 had acquired the organism, implying that there may have been an undetected environmental source or vector of transmission. The acquisition of the infection could also have been due to overcrowding of pod 1 where sufficient space was not provided between beds and proper hand hygiene was not maintained (26). Overall, Maragakis et al. (2008) believe that the organism was spread by transient carriage on the hands of healthcare personnel or on contaminated endotracheal respiratory care equipment.
In Vienna, Austria, another outbreak of S. marcescens was detected in a NICU between October 1, 2000, and February 28, 2001. After eight patients were found to be colonized with the bacterium, the NICU was closed on December 27 for 10 days; to implement infection control measures and thoroughly disinfect the care unit. The primary case had septicemia with S. marcescens and after analysis of risk factors this neonate was suspected to have acquired the bacterium from his mother, who was treated 9 days with cefuroxime as a prophylactic treatment to avoid chorioamnionitis (inflammation of fetus) and intrauterine infection of the neonate after premature rupture of the membrane (1). A second case located next to the primary case also was infected with S. marcescens conjunctivitis. All strains of S. marcescens were biologically identical, containing no pigmentation and were sensitive to imipenem S, cefotaxin R, cefpirom S, gentamicin S, amikacin S, trimethoprim S, and ciprofloxacin S. However, two cases were identified after the unit was closed and differed from the previous S. marcescens strains due to their red pigmentation and susceptibility of all antibiotics. To summarize, healthcare workers may have spread the bacterium after handling infected neonates and mothers may have been exposed to antibiotics that caused overgrowth of resistant organisms. This rapidly spreading infection was halted at an early stage with aggressive infection control measures(1).
Cases in Animals
Perez et al. (2011) reports nonhospital-acquired S. marcescens bacteria in a 12 year old male Dalmatian dog. The owners observed symptoms of a head tilt to the right, falling to the left, and nystagmus (involuntary eye movements). The primary veterinarian diagnosed the dog with canine idiopathic vestibular disease which affects neurological pathways. Five days later, the vestibular signs continued in the untreated dog along with vomiting. The dog was re-evaluated for a heart murmur and fever and referred to the Veterinary Teaching Hospital, College of Veterinary Medicine at North Carolina State University for additional diagnostic evaluation. An echocardiography found lesions on the aortic valve causing abnormal growth which was diagnosed as aortic valve endocarditis (Figure 5). Blood samples collected from different anatomical sites resulted in negative detection of Anaplasma spp., Borrelia burgdorferi, Ehrlichia canis antibodies, and Diofilaria immitis antigen. Subsequently, the dog was treated with amplicillin, sulbactam, and doxycycline antibiotics. In conjunction, famotidine and metoclopramide were administerd for the vomiting. Despite aggressive therapy for congestive heart failure, the dog failed to respond to antimicrobials, developed opisthotonus (body frame forms a reverse bow due to severe spasms and hyperextension), and died within six hours after admission. Necropsy confirmed aortic valve endocarditis and were consistent with heart failure and septicemia. The blood samples returned in less than 12 hours, yielding a heavy growth of a pure culture of S. marcescens. This bacterium was resistant to the treatment antibiotics, however; it was susceptible to quinolones, third generation cephalosporins, imipenem, and aminoglycosides. This case of S. marcescens accumulated rapidly within the dog who was not hospitalized prior to infection. Thus, the dog acquired this infection in a community-related setting (31).
Many other animal related cases have been described in hospital practices. The first documentation of nosocomial Serratia spp septicemia was reported in six dogs in 1973. Prior to Serratia spp septicemia diagnosis, all six dogs were previously diagnosed with debilitating diseases and later developed a fever under intensive hospital care with an indwelling jugular catheter (31, 41). Although the source of infection was not determined in these six cases, subsequent studies found that the high prevalence of S. marcescens in hospital related cases were due to contaminated benzalkonium chloride sponge pots used as an antiseptic.
A study from South Africa isolated 22 IV catheters from 100 dogs with parvoviral enteritis. Only 2 catheters tested positive for S. marcescens (22, 31). In another case, a Doberman Pinscher underwent a tooth extraction and later developed fatal necrotizing fasciitis in association with S. marcescens. It was suggested that the bacteria was transmitted in the oral surgery by skin contamination at injection sites (31, 33).
During the winter of 1952, a herd of twenty-four milking cattle were housed in a conventional type barn, where a mild outbreak of mastitis due to haemolytic Staphylococcus aureus occurred. In this community setting, they also isolated S. marcescens from incubated milk (Table 1). The cows’ symptoms included swelling of the gland and visibly abnormal milk clots. The organism was found to be susceptible to four or five grams of neomycin and was eliminated over a three day period (Table 1)(3).
Research
Many treatment methods have been limited by the emergence of multiple drug-resistant strains of S. marcescens. In one study, resveratrol (RSV; trans-3,5,4’-trihydroxystilbene) was studied to see if it has in vivo prophylactic or therapeutic potential in which it could possibly prevent and treat S. marcescens infections. RSV has many diverse biological effects including anti-cancer, anti-inflammation, anti-diabetics, and cancer chemoprevention. Chia-Chen Lu et al. (2008) evaluated the effects of RSV against pneumonia which is an infection characterized by cough with sputum production, high fever, and mortality of different degrees. The body initiates a response to the build-up of infectious particles by using alveolar macrophages (AV) to synthesize and secrete a wide array of pro-inflammatory proteins (cytokines and chemokines) in the cell. Rats were used as a model system to study acute pneumonia induced by instillation of S. marcescens. Figure 6 shows that the survival rate of animals was 67% without RSV pretreatment but was 83% for animals pretreated with RSV. Results showed that RSV pretreatment for 3 days increased AM infiltration, elevated NK cell activity, decreased bacterial load in lungs, and overall decreased mortality rate. Therefore, these results suggest that RVS might be beneficial as a preventative measure against patients with S. marcescens-induced acute pneumonia (23).
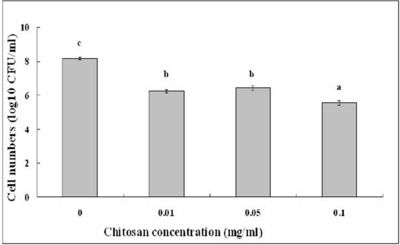
Li et al. (2011) studied S. marcescens isolated from an edible cactus plant and silkworms to evaluate the effects of the bactericidal activity of chitosan. Chitosan is industrially obtained by N-deactylation of shrimp and crapshell chitin. Previously, chitosan has shown a potential to control bacterial septicemic disease of silkworms caused by S. marcescens (19, 20). Along with chitosan being useful as a pharmaceutical agent in biomedicine, it is also useful as an antimicrobial compound in agriculture, a potential elicitor of plant defense responses, an additive in the food industry, and a hydrating agent in cosmetics. Chitosan exhibits antimicrobial activity against fungi, algae, and some bacteria. The antimicrobial uses of chitosan need to be further studied in depth because differences in its derivatives are affected by the degree of deacetylation, the molecular weight, the ionic strength, the pH, and the temperature (9). Li et al. (2011) conducted further research to examine the activity of chitosan against plant species affected with S. marcescens. The surviving cell numbers of the S. marcescens strain ZJ-S0801 decreased with increasing amounts of chitosan (Figure 7). Strong antimicrobial activity of chitosan was shown against plant associated S. marcescens. This research of chitosan will be useful in the control of contaminated fruit and vegetable products since the infected plant strain may play a possible role in human and animal food sources(19).
Conclusion
S. marcescens is an opportunistic pathogen causing a plethora of nosocomial infections in humans and some cases have been reported in animals. Many strains have become resistant to a variety of drugs. Future research, should evaluate different measures to prevent outbreaks of Serratia marcescens by evaluating antibiotics and the mode of entry into the bacterium. Pathogenic pathways should be more fully defined to understand how resistant S. marcescens infections may become vulnerable to different treatment methods.
References
[1]Assadian, Ojan, Berger, Angelika, Aspöck, Christoph, Mustafa, Stefan, Kohlauser, Christina, Hirschl, Alexander. 2002. Nosocomial Outbreak of Serratia marcescens in a Neonatal Intensive Care Unit. Infection control and Hospital Epidemiology 23: 457-463
[2]Bar-Ness, Ronit, and Rosenberg, Mel. 1989. Putative Role of 70 kDa Outer-surface Protein in Promoting Cell-surface Hydrophobicity of Serratia marcescensRZ. General Microbiology 135: 2277-2281
[3]Barnum, D.A., Thackeray, E.L., and Fish, N.A. 1958. An Outbreak of Mastitis Caused by Serratia marcescens. Canadian Journal of Comparative Medicine 22: 392-395
[4]Breed, R.S., Murray, E.G.D., and Smith, N.R. 1957.Bergey’s Manual of Determinative Bacteriology. 9th edition. Page 187
[5]Castelli, Maria E., Fedrigo, Griselda V., Clementín, Ana L., Ielmini, M. Verónica, Feldman, Mario F., and Véscovi, Eleonora G. 2008. Enterobacterial Common Antigen Integrity Is a Checkpoint for Flagellar Biogenesis in Serratia marcescens. Bacteriology 190: 213-220
[6]Chen, Jing, Kuroda, Teruo, Huda, Nazmul, Mizushima, Tohru, and Tsuchiya, Tomofusa. 2003. An RND-type multidrug efflux pump SdeXY from Serratia marcescens. Antimicrobial Chemotherapy 52: 176-179
[7]Clayton, Edwin, and Graevenitz, Alexander V. 1966. Nonpigmented Serratia marcescens. American Medical Association 197: 111-116
[8]Coulthurst, Sarah J., Williamson, Neil R., Harris, Abigail K.P., Spring, David R., and Salmond, George P.C. 2006. Metabolic and regulatory engineering of Serratia marcescens: mimicking phage-mediated horizontal acquisition of antibiotic biosynthesis and quorum-sensing capacities. Microbiology 152: 1899-1911
[9]Fineran, Peter C., Williamson, Neil R., Lilley, Kathryn S., and Salmond, George P.C. 2007. Virulence and Prodigiosin Antibiotic Biosynthesis in Serratia Are Regulated Pleiotropically by the GGDEF/EAL Domain Protein, PigX. Bacteriology 189: 7653-7662
[10]Haddix, Pryce L., Jones, Sarah, Patel, Pratik, Burnham, Sarah, Knights, Kaori, Powell, Joan N. and LaForm, Amber. 2008. Kinetic Analysis of Growth Rate, ATP, and Pigmentation Suggests an Energy-Spilling Function for the Prodigiosin of Serratia marcescens. Bacteriology 190: 7453-7463
[11]Hejazi, A. and Falkiner, F.R. 1997. Serratia marcescens. Medical Microbiology 46: 903-912
[12]Hornsey, Michael, Ellington, Matthew J., Doumith, Michel, Hudson, Sue, Livermore, David M., and Woodford, Neil. 2010. Tigecycline resistance in Serratia marcescens associated with up-regulation of the SdeXY-HasF efflux system also active against ciprofloxacin and cefpirome. Antimicrobial Chemotherapy 65: 479-482
[13]Kalivoda, Eric J., Stella, Nicholas A., O’Dee, Dawn M., Nau, Gerard J., and Shanks, Robert M.Q. 2008. The Cyclic AMP-Dependent Catabolite Repression System of Serratia marcescens Mediates Biofilm Formation through Regulation of Type 1 Fimbriae. Applied and Environmental Microbiology 74: 3461-3470
[14]Kida, Yutaka, Inoue, Hiroyoshi, Shimizu, Takashi, and Kuwano, Koichi. 2007. Serratia marcescens Serralysin Induces Inflammatory Responses through Protease-Activated Receptor 2. Infection and Immunity 75: 164-174
[15]Koh, Kai S., Lam, Kin W., Alhede, Morten, Queck, Shu Y., Labbate, Maurizio, Kjelleberg, Staffan, and Rice, Scott A. 2007. Phenotypic Diversification and Adaptation of Serratia marcescens MG1 Biofilm-Derived Morphotypes. Bacteriology 189: 119-130
[16]Kumar, Ayush, and Worobee, Elizabeth A. 2002. Fluoroquinolone resistance of Serratia marcescens: involvement of a proton gradient-dependent efflux pump. Antimicrobial Chemotherapy 50: 593-596
[17]Labbate, Maurizio, Zhu, Hua, Thung, Leena, Bandara, Rani, Larsen, Martin R., Willcox, Mark D.P., Givskov, Michael, Rice, Scott A., and Kjelleberg, Staffan. 2007. Quorum-Sensing Regulation of Adhesion in Serratia marcescens MG1 Is Suface Dependent. Bacteriology 189: 3461-3470
[18]Langrock, Marie-Lousie, Linde, Hans-Jörg, Landthaler, Michael, and Karrer, Sigrid. 2008. Leg ulcers and abscesses caused by Serratia marcescens. Eur J Dermatol 18: 705-707
[19]Li, Bin, Yu, Rongrong, Liu, Baoping, Tang, Qiaomei, Zhang, Guoqing, Wang, Yanli, Xie, Guanlin, Sun, Guochang. 2011. Characterization and comparison of Serratia marcescens isolated from edible cactus and from silkworm for virulence potential and chitosan susceptibility. Microbiology 42: 96-104
[20]Li, B., Xu, L.H., Chen, X.L., Lui, B.P., Zhu, B., Fang, Y., Qui, W., and Xie, G.L. 2009. Effect of chitosan solution on the bacterial septicemia disease of Bombyx mori (Lepidoptera: Bombycidae) caused by Serratia marcescens. Appl. Entomol. Zool. 45: 145-152
[21]Lima, KV, Carvalho,RG, Carneiro, IC, Lima, JL, Sousa Cde, O., Loureiro, EC, Sá, LL, and Bastos, FC. I 2011. Outbreak of neonatal infection by an endemic clone of Serratia marcescens. Revista da Sociedade Brasilleira de Medicina Tropical 44: 106-109
[22]Lobetti RG, Joubert KE, Picard J, et al. 2002. Bacterial colonization of intravenous catheters in youngs dogs suspected to have parvoviral enteritis. Am Vet Med Assoc 220: 1321-1324
[23]Lu, Chia-Chen, Lai, Hsin-Chih, Hsieh, Shang-Chen, and Chen, Jan-Kan. 2008. Resveratrol ameliorates Serratia marcescens induced acute pneumonia in rats. Leukocyte Biology 83: 1028-1037
[24]Makimura, Yutaka, Asai, Yasuyuki, Sugiyama, Akiko, and Ogawa, Tomohiko. 2007. Chemical structure and imunobiological activity of lipid A from Serratia marcescens LPS. Medical Microbiology 56: 1440-1446
[25]Mammeri, Hedi, Poirel, Laurent, Bemer, Pascal, Drugeon, Henri, and Nordmann, Patrice. 2004. Resistance to Cefepime and Cefpirome Due to a 4-Amino-Acid Deletion in the Chromosome-Encoded AmpC β-Lactamase of a Serratia marcescens Clinical Isolate. Antimicrobial Agents and Chemotherapy 48: 716-720
[26]Maragakis, Lisa L., Winkler, Amy, Tucker, Margaret G., Cosgrove, Sara E., Ross, Tracy, Lawson, Edward, Carroll, Karen C., Perl, Trish M. 2008. Outbreak of Multidrug-Resistant Serratia marcescens Infection in a Neonatal Intensive Care Unit. Infection Control and Hospital Epidemiology 29: 418-423
[27]Maseda, Hideaki, Hashida, Yumiko, Konaka, Rumi, Shirai, Akihiro, and Kourai, Hiroki. 2009. Mutational Upregulation of a Resistance-Nodulation-Cell Division-Type Multidrug Efflux Pump, SdeAB, upon Exposure to a Biocide, Cetylpyridinium Chloride, and Antibiotic Resistance in Serratia marcescens. Antimicrobial Agents and Chemotherapy 53: 5230-5235
[28]Matsuo, Taira, Chen, Jing, Minato, Yusuke, Ogawa, Wakano, Mizushima, Tohru, Kuroda, Teruo, and Tscuhiya, Tomofusa. 2008. SmdAB, a Heterodimeric ABC-Type Multidrug Efflux Pump, in Serratia marcescens. Bacteriology 190: 648-654
[29]Morohoshi, Tomohiro, Shiono, Toshitaka, Takidouchi, Kiyomi, Kato, Masashi, Kato, Norihiro, Kato, Junichi, and Ikeda, Tsukasa. 2007. Inhibition of Quorum Sensing in Serratia marcescens AS-1 by Synthetic Analogs of N-Acylhomoserine Lactone. Applied and Environmental Microbiology 73: 6339-6344
[30]Nicasio, Anthony M., Quintiliani Jr, Richard, DeRyke, C Andrew, Kuti, Joseph L., Nicolau, David P. 2007. Treatment of Serratia marcescens Meningitis with Prolonged Infusion of Meropenem. The Annals of Pharmacotherapy 41: 1077-1081
[31]Perez, Cristina, Fujii, Yoko, Fauls, Megan, Hummel, James, and Breitschwerdt, Edward. 2011. Fatal Aortic Endocarditis Associated with Community-Acquired Serratia marcescens Infection in a Dog. American Animal Hospital Association 47: 133-137
[32]Petty, Nicola K., Foulds, Ian J., Pradel, Elizabeth, Ewbank, Jonathan J., and Salmond George P.C. 2006. A generalized transducing phage (ϕIF3) for the genomically sequenced Serratia marcescens strain DB11: a tool for functional genomics of an opportunistic human pathogen. Microbiology 152: 1701-1708
[33]Plavec T, Zdovc I, Juntes P, et al. 2002. Necrotizing fasciitis caused by Serratia marcescens after tooth extraction in a Doberman Pinscher: a case report. Veterinarni Medicina 53: 629-635
[34]Quarles, John M., Belding, Ralph C., Beaman Cabrera, Teofila, and Gerhardt, Philipp. 1974. Hemodialysis Culture of Serratia marcescens in a Goat-Artificial Kidney-Fermentor System. Infection and Immunity 9: 550-558
[35]Rabea, Entsar I., Badaway, Mohamed E.T., Stevens, Christian V.m Smagghe, Guy,and Steurbaut, Walter. 2003. Chitosan as Antimicrobial agent: Applications and Mode of Action.Biomacromolecules 4: 1457-1465
[36]Ryu, Dong Jin, Oh, Sang Ho, Choi, Yoon Jin, and Lee, Ju Hee. 2010. A Case of Serratia marcescens After Augmentation Rhinoplasy. American Society for Dermatologic Surgery 36: 2079-2081
[37]Statham, Melissa M., Vohra, Amit, Mehta, Deepak K., Baker, Troy, Sarlay, Robert, and Rutter, Michael J. 2009.
Serratia marcescens causing cervical necrotizing oropharyngitis. International Jouranl of Pediatric Otorhinolaryngology 73: 467-473
[38]Trilla, A. 1994. Epidemiology of nosocomial infections in adult intensive care units. Intensive Care Med 20: S1-S4
[39]Voelz, Alexander, Müller, Andreas, Gillen, Julia, Le, Celine, Dresbach, Till, Engelhart, Steffen, Exner, Martin, Bates, Christine J., and Simon, Arne. 2010. Outbreaks of Serratia marcescens in neonatal and pediatric intensive care units: Clinical aspects, risk factors and management. Int. J. Hyg. Environ. Health 213: 79-87
[40]Vonberg, Weitzel-Kage, Behnke, and Gastmeier. 2011. Worldwide Outbreak Database: the largest collection of nosocomial outbreaks. Infection 39: 29-34
[41]Wilkins RJ. 1973. Serratia marcescens septicaemia in the dog. Small Anim Pract 14: 205-15
Edited by Brittany Currey,student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.
