Opportunistic Infections Caused by Serratia marcescens: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
[[Image: | [[Image:rhinoplasty.jpg|thumb|300px|right|A 0.5-cm protruding crusted papule on the tip on the nose infected with <br><i>Serratia marcescens</i>. Image from:http://onlinelibrary.wiley.com/doi/10.1111/j.1524-4725.2010.01788.x/pdf]] | ||
[[Image:hpylori_lg.jpg|thumb|300px|right|Figure 1. <i>Helicobacter pylori</i>. Image from:http://medgadget.com/archives/2005/10/the_nobel_prize.html]] | |||
<br>By [Brittany Currey]<br> | <br>By [Brittany Currey]<br> | ||
<br>At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of: | <br>At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of: | ||
Revision as of 20:44, 25 April 2011
Introduction
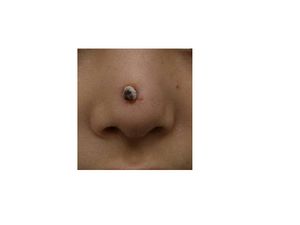
Serratia marcescens. Image from:http://onlinelibrary.wiley.com/doi/10.1111/j.1524-4725.2010.01788.x/pdf
By [Brittany Currey]
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Serratia marcescens, a rod-shaped gram-negative bacterium, is a member of the Enterobacteriaceae family. Ubiquitous in nature, S. marcescens is found on dead organic material, while some inhabit soil, water, air, plants, animals, or food. In particular, food sources that provide a nutrient rich environment include starchy variants (Hejazi and Falkiner 1997). Some of these bacteria can be identified in ecological niches by their red pigment, prodigiosin (2-methyl-3-penty-6-methoxyprodigiosin), which can resemble blood (cite). Since this pigment is easily recognized in certain strains due to its red coloring, it has been extensively used as a biological marker (Perez). Also, prodigiosin has antibacterial, antifungal, antiprotozoan, and immunosuppressant activity. Environmental signals such as temperature, phosphate limitation, and medium components regulate prodigiosin production. Prodigiosin is not the only secreted product, many more include protease, nucleases, lipase, chitinase, the biosurfactant serrawettin, and hemolysin (Morohoshi)
Although, S. marcescens was considered to be an innocuous, non-pathogenic organism, over the last two decades they have become an opportunist pathogen causing nosocomial infections (Hejazi). A broad range of hospital-acquired infections include respiratory tract infections, urinary tract infections (UTI), septicaemia, meningitis, pneumonia, conjunctivitis wound and eye infections, osteomyelitis, keratoconjunctivitis, keratitis, endophthalmitis and endocarditis (Hejazi, Matsuo, Castelli, Kida, Koh). S. marcescens are also capable of causing disease in a diverse group of organisms including animals, coral, insects, and plants (Hejazi and Coulthurst)
Many problems arise in treating nosocomial infections because of resistance to a variety of antibiotics, such as cephalosporins, aztreonam, imipenem, cefotaxime, and ceftazidime (Kumar, Mammeri). Therefore, novel treatment techniques are in need to eliminate infections without overuse of the antibiotic (Morohoshi).
Rare cases of S. marcescens include nonhospital associated infections. These cases are linked to patients with immune deficiencies or chronic debilitating diseases. Human medicine, outside of hospital settings, can involve intravenous drug use that can also spread S. marcescens. (Perez).
Section 1
Include some current research, with at least one figure showing data.
Cases in Humans
First cases of nosocomial infections were recognized in humans in 1951 (perez). A 54-year-old healthy female developed a very rare case of Serratia marcescen infection on the face. The infection consisted of a slightly elevated, pea-sized crusted plaque surrounded by reddish halo on her left cheek. Tissue cultures showed that Serratia marcescens was sensitive to levofloxacin and ciprofloxacin (Jornet). Other Cutaneous infections caused by S. marcescens are uncommon but may be predisposed by immunocompromised conditions or pre-damaged skin (Langrock). Langrock et al. (2008) presented a case in which a 73-year-old man contained multiple ulcers and painful nodules on the lower right leg as well as abscesses on the right malleolous lateralis. Skin cultures taken from the lesions were highly positive for S. marcescens. The skin lesions had appeared 4 weeks and neither topical nor oral antibiotic treatment with pencillin improved the lesions. Instead, topical therapy, repeated incision and draining of the abscesses, as well as the continuation of intravenous ertapenem improved skin lesions. The lesions improved gradually after 10 days of treatment and infection ceased completely, leaving hyperpigmented scars on the lower right leg.
Other cases of Serratia marcescens infecting adults include intravenous catheters as a common primary source of bloodstream infection (Pien). Five cases have been reported of Necrotizing fasciitis (NF) which infects soft tissues that spreads along fascia lines. Four of the five cases had lower extremity NF while one case involved an immunocompetent 2-year-old-who developed cervical NF infecting fascia planes of the head and neck (Statham). This patient was treated with serial debridements with irrigation of 3% hydrogen peroxide that was effective at removing necrotic tissue without need for external incisions. Unfornately, this patient was in an intensive care unit for a prolonged period of time due to sedation to tolerate wound packing and rapidly succumbed to the infection (Statham and Newton).
Several outbreaks of Serratia marcescens have been found in neonatal and pediatric intensive care units (NICU, PICU) throughout the world. Voelz et al. (2010) reported 27 studies in which 34 outbreaks of S. marcescens resulted. The Department of Hospital Epidemiology and Infection Control at the Johns Hopkins Hospital in Baltimore, Maryland, detected an outbreak of multidrug–resistant (MDR) S. marcescens in a 36-bed NICU. The investigation took place from October 2004 through February 2005 in which one-third of the neonates hospitalized became infected or colonized the MDR bacterium. The strain of S. marcescens was resistant to a majority of commonly prescribed antimicrobial agents. The epidemiologic report did not identify environmental or healthcare worker hands as the source of contamination because the case-control study was limited by a small sample size. However, individuals with low birth rates have previously been found as a risk factor for S. marcescens infection among neonates. Also, transmission of MDR S. marcescens was thought to be acquired by the location within the NICU. Here, reports show that the majority of the patients in pod 1 had acquired the organism, implying that there may have been an undetected environmental source or vector of transmission. The acquisition of the infection could also have been due to overcrowding of pod 1 where sufficient space was not provided between beds and improper hand hygiene (Maragakis 2008). Overall, Maragakis et al. (2008) believe that the organism was spread by transient carriage on the hands of healthcare personnel or on contaminated endotracheal respiratory care equipment.
In Vienna, Austria, another outbreak of S. marcescens was detected in a NICU between October 1, 2000, and February 28, 2001. After eight patients were found to be colonized with the bacterium, the NICU was closed on December 27 for 10 days; to implement infection control measures and thoroughly disinfect the care unit. The primary case had septicemia with S. marcescens and after analysis of risk factors this neonate was suspected to have acquired the bacterium from his mother, who was treated 9 days with cefuroxime as a prophylactic treatment to avoid chorioamnionitis (inflammation of fetus) and intrauterine infection of the neonate after premature rupture of the membrane (Assadian). A second case located next to the primary case also was infected with S. marcescens with conjunctivitis. All strains of S. marcescens were biologically identical, containing no pigmentation and were sensitive to imipenem S, cefotaxin R, cefpirom S, gentamicin S, amikacin S, trimethoprim S, and ciprofloxacin S. However, two cases were identified after the unit was closed and differed from the previous S. marcescens strains due to their red pigmentation and susceptibility of all antibiotics. To summarize, affected neonates may have spread the infection via horizontal transmission by healthcare workers and mothers may have been exposed to antibiotics that caused overgrowth of resistant organisms. This rapid spreading infection was halted at an early stage with aggressive infection control measures.
Cases in Animals
Perez et al. (2011) reports nonhospital-acquired S. marcescens bacteria in a 12 year old male Dalmatian dog. The owners observed symptoms of a head tilt to the right, falling to the left, and nystagmus (involuntary eye movements). The primary veterinarian diagnosed the dog with canine idiopathic vestibular disease which affects neurological pathways. Five days later, the vestibular signs continued in the untreated dog along with vomiting. The dog was re-evaluated for a heart murmur and fever and referred to the Veterinary Teaching Hospital, College of Veterinary Medicine at North Carolina State University for additional diagnostic evaluation. An echocardiography found lesions on the aortic valve causing abnormal growth which was diagnosed as aortic valve endocarditis. Blood samples collected from different anatomical sites resulted in negative detection of Anaplasma spp., Borrelia burgdorferi, Ehrlichia canis antibodies, and Diofilaria immitis antigen. Subsequently, the dog was treated with amplicillin sulbactam and doxycycline antibiotics. In conjunction, famotidine and metoclopramide were administerd for the vomiting. Despite aggressive therapy for congestive heart failure, the dog failed to respond to antimicrobials, developed opisthotonus (body frame forms a reverse bow due to severe spasms and hyperextension), and died within six hours after admission. Necropsy confirmed aortic valve endocarditis and were consistent with heart failure and septicemia. The blood samples returned in less than 12 hours, yielding a heavy growth of a pure culture of S. marcescens. This bacterium was resistant to the treatment antibiotics, however; it was susceptible to quinolones, third generation cephalosporins, imipenem, and aminoglycosides. This case of S. marcencens accumulated rapidly within the dog who was not hospitalized prior to infection. Thus, the dog acquired this infection in a community-related setting (perez).
Many other animal related cases have been described in hospital practices. For instance, six dogs were infected and this was the first documentation of nosocomial Serratia spp septicemia in 1973. Prior to Serratia spp septicemia diagnosis, all six dogs were previously diagnosed with debilitating diseases and later developed a fever under intensive hospital care with an indwelling jugular catheter (Wilkins cited in Perez). Although, the source of infection was not determined in these six cases, subsequent studies found that the high prevalence of S. marcescens in hospital related cases were due to contaminated benzalkonium chloride sponge pots used as an antiseptic.
A study from South Africa isolated 22 IV catheters from 100 dogs with parvoviral enteritis. Only 2 catheters tested positive for S marcescens (Lobetti cited in Perez). In another case, a Doberman Pinscher underwent a tooth extraction and later developed fatal necrotizing fasciitis in association with S. marcescens. It was suggested that the bacteria was transmitted in the oral surgery by skin contamination at injection sites (Plavec cited in Perez).
Conclusion
Overall text length at least 3,000 words, with at least 3 figures.
References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.
