Rhodothermus marinus: Difference between revisions
| Line 3: | Line 3: | ||
Domain: Bacteria; Phylum: Bacteroidetes; Class: Bacteroidetes; Order: Bacteroidetes order II Incertae sedis; Family: Rhodothermaceae; Genus: Rhodothermus | Domain: Bacteria; Phylum: Bacteroidetes; Class: Bacteroidetes; Order: Bacteroidetes order II Incertae sedis; Family: Rhodothermaceae; Genus: Rhodothermus | ||
[[Image:Phylogenetic tree of R. marinus.png|thumb|200px|right|Phylogenetic tree highlighting the position of R. marinus strain R-10T relative to S. ruber, the second species within the ‘Rhodothermaceaea’.]]] | |||
==Description and Significance== | ==Description and Significance== | ||
Revision as of 15:07, 10 April 2012
Classification
Domain: Bacteria; Phylum: Bacteroidetes; Class: Bacteroidetes; Order: Bacteroidetes order II Incertae sedis; Family: Rhodothermaceae; Genus: Rhodothermus
]
Description and Significance

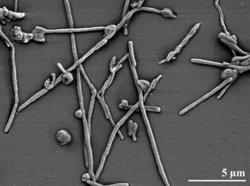
R. Marinus form reddish-coloured, low convex colonies, 3-4 mm in diameter and with an entire edge. The bacteria also has Gram negative rods about 0.5 µm in diameter and 2-2.5 µm long.
R. Marinus can be found in several similar but distantly located geothermal habitats. Many have large fluctuations in environmental conditions. Temperatures higher than 65 ̊C allow for optimum activity and growth. Shallow water hot springs of Iceland have been a place for studying samples of R. Marinus. R. Marinus has also been found in other regions of the world including Portugal, Italy and Monserrat Island in the Caribbean Sea.
Most thermophiles that have been studied in the past have been found in deep-sea hydrothermal vents in the Pacific Ocean. The study of R. Marinus found in shallow- water hot springs in comparison to similar thermophiles found deep in the ocean can bring more understanding to these organisms’ ability to survive in extreme environments.
Genome Structure
Complete genome sequencing of Rhodothermus marinus (strain R-10=DSM4252=ATCC43812) is a part of the Genomic Encyclopedia of Bacteria and Archaea project. This is the first time to obtain the complete genome sequence of the genus Rhodothermus, and the second sequence from members of the family Rhodothermaceae. The whole genome (3,386,737 bp) includes one main circular chromosome and one circular plasmid. The GC content is 64.3%, and the DNA coding region is about 92.5%. The length of the plasmid is 125 kbp. There are 2,914 protein-coding and 48 RNA genes among the total 2,962 predicted genes. Only 71.6% (2,122) genes were identified with a putative function. However, the other genes are designated as hypothetical proteins. Moreover, there were 51 pseudogenes identified. The genome project is deposited in the Genomes Online Database, and the complete genome sequence in Genebank.
Cell Structure, Metabolism and Life Cycle
According to Batista et al, Rhodothermus marinus has a complex I structure, which consists of fourteen subunits. Seven are hydrophilic proteins located in the peripheral arm, the other 7 hydrophobic proteins in the membrane arm. There were many protein and peptide separation strategies. The combination of all of the methodologies allowed the identification of complex I canonical subunits, there was also a possible novel subunit, a PCD (pterin-4[alpha]-carbinolamine dehydratase). PCD possibly plays a regulatory role, though further research needs to be conducted.
Two citrate synthases have been identified and purified from R. marinus. Both are thermostable. One is a hexameric protein and the first thermostrable, hexameric citrate synthase that has been isolated. The second is a dimeric enzyme, it has both citrate synthase and 2-methyl citrate synthase activities. Propionyl-coenzyme A is used in 2-methyl citrate synthase as one of the substrates. It is not used as the sole carbon source because no growth can be observed with it alone (Karlsson et al).
Ecology
Habitat
R. marinus was first isolated from submarine alkaline freshwater hot springs in Isafjardardjup, Iceland in 1988 and from other geothermal sites in Iceland including coastal springs from a borehole effluent in Oxarfjordur, Iceland, from borehole effluents from a powerplant at the Blue Lagoon, and from a salt factory in Reykjanes, Iceland. As well, it has been isolated from similar habitats in geothermal environments at various regions around the world, including Praia de Ribeira Quenta, Ferraria, Sa˜o Miguel in the Azores, Portugal, Stufe di Nerone near Naples, Italy, and on Monserrat island in the Carribean Sea. It should be noted that the populations of R. marinus in these geographic locations are genetically distinct. High genetic diversity and low horizontal gene transfer indicate the populations at these geographically distinct sites are evolving independently and indicate that genetic drift and geographic isolation is an important mechanism in species divergence (Petursdottir et al).
R. marinus is a heterotrophic obligate aerobe that is thermophilic and slightly although strictly halophilic. The growth of this organism is dependent on a multitude of factors including temperature, salt and oxygen concentration, and the availability of organic material. Growth occurs at a temperature range of 54-77 degrees Celsius with optimal growth occurring at 65 degrees Celsius. R. marinus has also been characterized at growing at NaCl concentrations optimally at 2% and not above 6%. This species only utilizes a single carbon source, with slight differences in carbon sources between strains. The physiology of this microbe dictates a narrow niche for it to occupy. R. marinus can only grow close to the openings of hot springs where oxygen is readily available and close to the surface of the water where the temperatures are warm.
Symbiosis
Not much is known about any symbiotic relationships that R. marinus might hold. Hopefully future ecological studies can shed light on such relationships.
Biogeochemical Significance
Canfield et al. characterized a cryptic sulfur cycle in oxygen-minimum zone waters off the Chilean coast. The biogeochemistry and microbial ecology of oxygen-minimum zones in marine environments were thought to have been dominated by nitrogen cycling. Through molecular techniques and rate measurements, Canfield showed that sulfate reduction and sulfide oxidation contribute to the energy flux and nutrient cycling in these waters. Dubbed the cryptic sulfer cycle, it has been linked to anammox and other nitrogen cycling processes. This suggests it may influence biogeochemical cycling in global oceans. During their study, they characterized the microbial community of these areas and Rhodotherms marinus was found using culture independent methods. However, its presence was not explained in the discussion. The presence of R. marinus in this environment is puzzling, as it is an obligate aerobe and requires specific factors for growth that may not be met in this environment. Hopefully future studies will elucidate the role of R. marinus in this process.
Environmental Importance
Rhodothermus marinus is important to the environment because it decomposes organic matter, releasing carbon, nitrogen, phosphorus, sulfur and other important nutrients back into the environment. It is also biotechnologically important as it has many thermostable enzymes that are used in research.
References
Author
Page authored by _____, student of Prof. Jay Lennon at Michigan State University.
<-- Do not remove this line-->