Thalassiosira pseudonana: Difference between revisions
| Line 45: | Line 45: | ||
4. Hamm, C.E., Merkel, R., Springer, O., Jurkojc, P., Maier, C., Smetacek, V. "Architecture and material properties of diatom shells provide effective mechanical protection." ''Nature.'' Vol. 421, pp. 841-843. | 4. Hamm, C.E., Merkel, R., Springer, O., Jurkojc, P., Maier, C., Smetacek, V. "Architecture and material properties of diatom shells provide effective mechanical protection." ''Nature.'' Vol. 421, pp. 841-843. | ||
5. Paasche, E. "Silicon and the ecology of marine plankton diatoms. I. Thalassiosira pseudonana (Cyclotella nana) grown in a chemostat with silicate as limiting nutrient." ''Marine Biology.'' Vol. 19, pp. 117-126. | |||
==Author== | ==Author== | ||
Revision as of 23:07, 28 April 2014
Classification
Eukaryota; Bacillariophyta; Coscinodiscophyceae; Thalassiosirales; Thalassiosiraceae [Others may be used. Use NCBI link to find]
pseudonana
|
NCBI: Taxonomy |
Thalassiosira pseudonana
Description and Significance
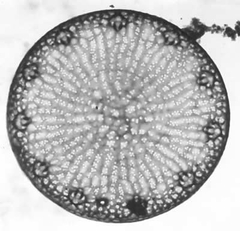
Thalassiosira psuedonana is a marine centric diatom. Diatoms are unicellular, eukaryotic,phytoplankton that display a unique evolutionary history and provide major ecological contributions in marine environments. Diatoms are capable of photosynthesis, having acquired plastids through secondary endosymbiosis of primary endosymbionts, including plants and, green,red, and glaucophyte algae. Having evolved 91.5 million years ago during the Upper Turonian period [1], analyses of these organisms display long-term contributions to deposits of diatomite, carbon cycling, global climate, and petroleum reserves. Today diatoms continue to have major ecological implications and are the most important marine phytoplankton in the oceans, generating up to 40% (45 to 50 billion metric tons) of the marine organic carbon produced each year [2]. These diatoms continue to play a fundamental role in global carbon cycling and global climate by influencing the flow of carbon dioxide in the oceans. As a result of the ecological importance of diatoms, T. pseudonana was the first diatom to undergo full genome sequencing. In addition, T. pseudonana have elaborate silicified cell wall nanostructures that may contribute to future study of silica nanotechnology. T. pseudonana diatoms display a unique combination of metabolic processes including genes for nitrogen fixation, the urea cycle, carbon fixation, iron uptake, and photosynthesis [3].
Genome Structure
The genome of T. pseudonana was sequenced by E. Armbrust et. al using a whole-genome shotgun approach. The nuclear genome of T. pseudonana is 34.5 million bp, accounting for a predicted total of 11,242 protein-encoding genes. The nuclear genome encodes 24 pairs of chromosomes, totaling 34.5 Mb. The plastid genome is 128,813 bp accounting for 144 protein-encoding genes. The mitochondrial genome is 43,287 bp with 40 protein-coding genes. The most abundant domain in T. pseudonana is protein kinase 1. T. pseudonana differs from many eukaryotic species and has a relatively low reliance on receptor kinases and leucine-rich receptor (LRR) containing receptors. The major transcription factor in T. pseudonana is the heat-shock family, which is also relatively uncommon in eukaryotic species. Suprisingly, of all horizontally acquired genes, T. pseudonana displays 865 proteins that align only with plants and only 182 proteins that align only with algae [3]. Genome analysis does not reveal recent large-scale transfers of plastid or mitochondiral DNA to the nuclear genome. There is evidence for an in-proccess transfer from plastid to nucleus of a psbW gene that encodes a photosytem II [3].
Cell Structure, Metabolism and Life Cycle
The frustule, or cell wall, of T. pseudonana is composed of hydrated silicon dioxide and a small amount of organic matter [3]. The frustule of diatoms can withstand extreme force, in some cases displaying resistance up to 720 µN. The rigidity of the frustules has resulted from an evolutionary co-arms race between diatoms and their predators, including copepods and euphausiids. These predators have silica-lined mandibles and gizzards lined with rows of sharp teeth [4]. Diatom frustules have evolved resistance to high external force as a result of the selective pressures posed by the architecture of their predators.
Silicon is required silicon for frustule formation. As a result, the rate of cell growth for planktonic T. pseudonana is limited by the availability of dissolved silicon in marine environments [5]. When T. pseudonana are grown in environments of reduced silicate concentrations result in loss of structure, specifically in the areola pattern near the center valve [5]. The process of generating and maintaining frustules controls biogenic silca cycling as diatoms take up silisalic acid from sea water to build their frustules [5]. After cell death biogenic silica that is not dissolves settles in the marine sediment and re-enters geological cycling [5].
T. pseudonana is capable of metabolizing multiple forms of nitrogen. T. pseudonana contains multiple transporter genes for inorganic forms of nitrogen including, nitrate, ammonium, phosphate, sulfate, and salicylic acid. T. pseudonana also contains genes that allow it to take up organic forms of nitrogen and catabolize amino acids [1]. Not present in any other eukaryotic photoautotrophs, T. pseudonana contains all enzymes necessary for a complete urea cycle. T. pseudonana carry out the urea cycle and the byproducts produced at various steps are distributed to other pathways, such as the synthesis of long-chain polyamines required for frustule formation [1].
Carbon fixation is an important metabolic process carried out by T. pseudonana due to its role in global geological carbon cycling. Genome analysis indicates that carbon fixation occurs in the cytoplasm of this organism, rather than in the plastid [1]. The decarboxylating enzymes necessary for the delivery of CO2 to rubisco are found in the cytoplasm and do not appear to be present within plastids [1].
Analysis of the genome of T. pseudonana reveals that diatoms absorb red and blue light, but not green light [1]. These diatoms possess homologs for cryptochromes, which absorb blue light, and homologs for phytochrome, which absorbs red light. Blue and red light are found most frequently at the water surface whereas green light penetrates deep within ocean water, suggesting that diatoms only encode photoreceptors for blue and red light to maintain close proximity to the water surface [1].
Ecology
T. pseudonana is a diatom found in marine ecosystems, including oceans and freshwater [1]. Through the processes of carbon fixation and photosynthesis,diatoms play a major role in the flux of atomspheric carbon dioxide in oceans [1]. Diatoms contribute 40% of organic carbon produced in the ocean each year and play a fundamental role in global carbon cycles.
T. pseudonana control biogenic silica processing to a great extent, such that all silicon atoms entering the oceans are incorporated into diatom frustules 40 times before entering the ocean floor [1]. The process of generating and maintaining frustules controls biogenic silca cycling as diatoms take up silisalic acid from sea water to build their frustules [5]. After cell death biogenic silica that is not dissolved in ocean waters settles in the marine sediment and re-enters geological cycling [5].
T. pseudonana has obtained photosynthesis capabilities through secondary endosymbiosis of organisms that had previously evolved photosynthesis capabilities through the uptake of chloroplast. Specifically, the genome of T. pseudonana displays homology with 182 proteins of the red algae, C. merolae. In addition, T. pseudonana displays homology with 865 proteins of genome of the plant, A. thaliana [1]. This diatom also shares homology with animals and cyanobacteria. The genome displays evidence of high levels of gene transfer between genomes during endosymbiosis establishment.
References
1. Sinninghe Damste, Jaap S., Muyzer, Gerard, Abbas, Ben, Rampen, Sebastian W., Masse, Guillaume, Allard, W. Guy, Belt, Simon T., Robert, Jean-Michel, Rowland, Steven J., Moldowan, J. Michael, Barbanti, Silvana M., Fago, Frederick J., Denisevich, Peter, Dahl, Jeremey, Trindade, Luiz A.F., Schouten, Stefan. "The Rise of the Rhizosolenid Diatoms." Science. 23 April 2004. Vol. 304, pp. 584-587.
2.Nelson, DM, Treguer, P, Brezinski MA, Leynaert, A, Queginer, B. "Production and Dissolution of Biogenic Silica in the Ocean - Revised Global Estimates, Comparsion with Regional Data and Relationship to Biogenic Sedimentation." Global Biogeochemical Cycles. September 1995. Vol. 9, pp. 359-372.
3. Armbrust, E. Virginia, Berges, John A., Bowler, Chris, Green, Beverly R., Martinez, Diego, Putnam, Nicholas H., Zhou, Shigou, Allen, Andrew E., Apt, Kirk E., Brzezinski, Mark A., Chaal, Balbir K., Chiovitti, Anthony, Davis, Aubrey K., Demarest, Mark S., Detter, J. Chris, Glavina, Tijana, Kapitonov, Vladimir V., Kroger, Nils, Lau, Winnie W.Y., Lane, Todd W., Larimer, Frank W., Lippmeier, J. Casey, Lucas, Susan, Medina, Monica, Montsant, Anton, Obornik, Miroslav, Parker, Micaela Schnitzler, Palenik, Brian, Pazour, Gregory J., Richardson, Paul M., Rynearson, Tatiana A., Saito, Mak A., Schwartz, David C., Thamatrakoln, Kimberlee, Valentine Klaus, Vardi, Assaf, Wilkerson, Frances P., and Rokhsar, Daniel S. "The Genome of the Diatom Thalassiosira pseudonana: Ecology, Evolution, and Metabolism." Science. 4 October 2004. Vol. 306, pp. 79-86.
4. Hamm, C.E., Merkel, R., Springer, O., Jurkojc, P., Maier, C., Smetacek, V. "Architecture and material properties of diatom shells provide effective mechanical protection." Nature. Vol. 421, pp. 841-843.
5. Paasche, E. "Silicon and the ecology of marine plankton diatoms. I. Thalassiosira pseudonana (Cyclotella nana) grown in a chemostat with silicate as limiting nutrient." Marine Biology. Vol. 19, pp. 117-126.
Author
Page authored by Kayla mitman, student of Prof. Jay Lennon at IndianaUniversity.