Clostridium as a Cancer Therapy: Difference between revisions
Anh Tran16 (talk | contribs) |
Anh Tran16 (talk | contribs) |
||
| Line 28: | Line 28: | ||
==Research and Studies== | ==Research and Studies== | ||
A team of researchers at John Hopkins has since revisited the idea of using microbes to treat cancer, and specifically studying ''Clostridium'' bacteria. In a study recently published in [http://en.wikipedia.org/wiki/Science_%28magazine%29 ''Science''], they used C. noyvi by removing the α-toxin-producing genes to make it safer for internal use. The new bacteria strain is called ''C. noyvi-NT'' (''C. noyvi-non-toxic'').[[#References |<sup>15</sup>]] When injected intravenously, ''C. novyi-NT'' spores produced substantial anti-tumor effects in experimental animals without excessive toxicity.[[#References |<sup>2</sup>]] | A team of researchers at John Hopkins has since revisited the idea of using microbes to treat cancer, and specifically studying ''Clostridium'' bacteria. In a study recently published in [http://en.wikipedia.org/wiki/Science_%28magazine%29 ''Science''], they used ''C. noyvi'' by removing the α-toxin-producing genes to make it safer for internal use. The new bacteria strain is called ''C. noyvi-NT'' (''C. noyvi-non-toxic'').[[#References |<sup>15</sup>]] When injected intravenously, ''C. novyi-NT'' spores produced substantial anti-tumor effects in experimental animals without excessive toxicity.[[#References |<sup>2</sup>]] | ||
===Intratumoral injection of ''Clostridium novyi-NT'' spores === | ===Intratumoral injection of ''Clostridium novyi-NT'' spores === | ||
Latest revision as of 17:28, 11 April 2015
Cancer is characterized by uncontrolled cell growth that forms tumors composed of necrotic, hypoxic, and/or well-oxygenated tissues1. In particular, the hypoxic regions in tumors are difficult to treat and are a major cause of treatment failures.8 Most conventional anticancer therapies only target the well-vascularized areas of tumors and rely on tumor vasculature or oxygen transport, leaving behind hypoxic cells that can potentially regenerate the tumor following treatment. Hypoxic regions are more resistant to systemic anticancer agents and radiotherapy, as oxygen is a required effector of radiation-induced cell death. The dose required to kill hypoxic cells through radiation is up to 3 times higher than the amount normally used in well-oxygenated cells.1 In search of a more effective and specific cancer targeting treatment, researchers have tested the use of bacteria for creating new cancer therapies. Studies have shown that bacteria-directed cancer treatments increase the rate of tumor reduction and only target cancer cells, leaving the other cells intact.12
One potential anticancer candidate currently being studied is Clostridium. Clostridium is one of the largest prokaryotic genera and consists of a diverse range of obligatory anaerobic bacteria. There are over 100 species of Clostridium, including common free-living bacteria and pathogens. Clostridium bacteria are Gram-positive and rod-shaped. Most members of the genus are motile and flagellated, and form oval or spherical endospores that distend from the cell.18
The endospores produced by Clostridium are highly resistant to extreme environmental conditions such as high temperatures, disinfectants, and low-energy radiation, and makes many pathogenic Clostridium bacteria a major health concern and of clinical significance. Several pathogens-producing potent toxins in this genera include C. tetani (tetanus), C. botulinum (food poisoning), and C. perfringens (gas gangrene)1. Another species, Clostridium novyi has been associated with an outbreak of serious illness and death amongst intravenous drug users. Despite pathogenic properties, research has shown that Clostridium novyi seems to be very promising in the fight against cancer as a bacteriolytic anti-cancer agent. It has been well known that severe bacterial infections can sometimes cure cancer patients once the bacterium’s pathogenicity is eliminated.14 17
History
The introduction of bacterial applications in cancer therapy is not an entirely novel idea as studies have shown that bacterial infections can sometimes cure cancer patients once the bacterium’s pathogenicity is eliminated. Using bacteria to treat cancer was first suggested by William B. Coley, who in 1891, injected Streptococcus pyogenes into a patient with terminal cancer.10 He thought that the induced infection would shrink the malignant tumor and was successful as he continued to inject more than 1000 cancer patients with dead bacteria or bacterial products over the next forty years.10 However, with the evolution of radiotherapy, chemotherapy, and other modern cancer treatments, the idea of using bacteria in cancer therapies were disregarded due to the inability to produce a viable anticancer agent, in part because of poor reproducibility and unacceptable toxicity.5 12 Since Coley’s original work, a variety of anaerobic bacterial species have been considered for cancer therapy.
Methods
Traditional cancer therapies
Current and traditional cancer therapies involve regulating cell division and growth of tumor cells at the genetic level. Conventional methods involve delivering tumor suppressor genes to cancer cells, delivering genes that activate toxic products to kills tumor cells and their neighbors, introducing defective genes that cause cell death, and directly attacking tumor cells and harnessing the immune responses to tumor antigens.13 However, the primary problem with many of these traditional methods is the lack of tumor specificity. Disadvantages of both viral and non-viral gene delivery methods include but are not limited to low transfection transduction levels, immune responses that may hinder repeated administrations of the vector, potential random integrations from retroviruses that could have carcinogenic effects, and a preferential infection of only non-dividing cells.1 Most drugs and current treatments are effective against rapidly dividing tumor cells but not against hypoxic regions in which cell proliferation is decreased.
Bacteria-directed cancer therapies
Difficulties in delivering anticancer chemotherapeutics to the poorly vascularized, hypoxic regions of tumors can be overcome with bacteria-based tumor specific therapies using anaerobic bacteria.13 Anaerobic bacteria can specifically target the hypoxic or necrotic regions of the tumors and can either directly exert innate oncolytic effects or act as a vector to introduce locally expressed therapeutic proteins.4 12 Bacteriolytic anti-cancer therapies use attenuated bacterial strains that are capable of selectively proliferating within tumors.9 An advantage of using bacteria to treat cancer is that bacteria can be modified relatively easily, such as by eliminating its toxicity or by equipping it with other therapeutic agents.7 Once a bacterium's toxicity has been eliminated, it can grow within a tumor without harming the host. In addition, some non-recombinant bacteria already exert inherent anti-tumor activities, and recombinant bacteria can be used as vectors to produce protein of therapeutic interest in tumor environments as an alternative to gene therapy.15 Using bacteria as a therapeutic agent can induce a strong immune response against tumor cells.11 Through the bacterial-directed protein delivery system, there will be an efficient distribution of vector throughout the tumor mass with sufficient transfection levels and transient gene expression. This method prevents the random insertion of foreign DNA into the genome and the transfection of tumor cells with therapeutic genes that may instead enhance anti-tumor properties.16 The bacteria can then be inactivated at any moment during therapy by administering antibiotics.4 13 The use of anaerobic bacteria is recently being studied in its ability to target oxygen-poor tumors.16
Clostridium as a Cancer Treatment
Due to their anaerobic nature, species of Clostridium bacteria have been recognized for their ability to selectively target and lyse tumor cells growing in hypoxic environments marked by uncontrolled growth and low oxygen levels.8 17 Intravenous administration of nonpathogenic bacterial strains or spores from nonpathogenic strains of Clostridium bacteria can serve as a selective tumor killer of areas overlooked by conventional cancer therapies.
In particular, Clostridium novyi is a highly mobile spore-forming organism that is extremely sensitive to oxygen and has never been shown to germinate in areas of tumors outside of the hypoxic regions.6 14 The bacteria are exquisitely sensitive to oxygen but its spores are stable to oxygen as well as to harsh conditions.3 Due to its highly motile nature, the bacteria can easily disperse itself throughout the tumor.14
Research and Studies
A team of researchers at John Hopkins has since revisited the idea of using microbes to treat cancer, and specifically studying Clostridium bacteria. In a study recently published in Science, they used C. noyvi by removing the α-toxin-producing genes to make it safer for internal use. The new bacteria strain is called C. noyvi-NT (C. noyvi-non-toxic).15 When injected intravenously, C. novyi-NT spores produced substantial anti-tumor effects in experimental animals without excessive toxicity.2
Intratumoral injection of Clostridium novyi-NT spores
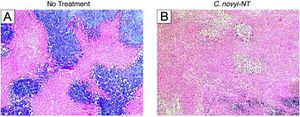
Reprinted from Long H. Dang et al. Combination bacteriolytic therapy for the treatment of experimental tumors. PNAS 2001;98:15155-15160 ©2001 by National Academy of Sciences
To study the effects of C. noyvi-NT as an anticancer agent, Roberts et al. (2014) injected a single dose of intravenous C. novyi-NT spores directly into mice and rabbits bearing transplanted tumors. An antitumor response was observed, including localized tumor necrosis, intense inflammatory responses, and complete responses in 25-30% of the treated animals.15
In the same experiment, researchers then intravenously injected C. novyi-NT spores into spontaneously occurring canine tumors to observe and to compare the effects across different species. For this experiment, each dog received at least one single intratumoral injection of 1×108 C. novyi-NT spores into one target tumor. Dogs received up to four cycles of treatment with a one-week interval between cycles, and were followed for at least 90 days after the first injection. The injections of C. novyi-NT spores were well-tolerated in the dogs, and the side effects were expected symptoms associated with bacterial infections mild in nature. Responses were observed in 37.5% of the 16 dogs, but complete responses were observed in only 3 of the dogs.15 Based on the overall success with improved survival rates in the experimental animals, researchers decided to further test the effects of intratumoral injection of Clostridium novyi-NT spores on human patients.
A phase 1 investigational study was initiated in human patients with solid tumors that were resistant to standard therapy. The first patient enrolled in this trial was a 53-year-old female diagnosed with advanced retroperitoneal leiomyosarcoma, a rare form of cancer resistant to chemotherapy and radiation. The patient was treated with an intratumoral injection of 10,000 C. novyi-NT spores. Extensive tumor necrosis of residual tumor cells was observed with reduced tumor tissue within and surrounding the bone.15
In another study of C. novyi-NT intravenous injection, spores were intravenously injected into mice bearing human colorectal cancer xenografts. Germination occurred exclusively within the tumors, and bacteria were dispersed throughout the tumor. The tumor tissue showed a significant reduction overtime following the intravenous injection.3 However, a rim of viable tumor was almost always left after treatment with the spores, limiting the efficacy and duration of the therapeutic response.3
Experimental results collectively support the further development of intratumoral injections of C. novyi-NT spores as a therapeutic for patients with locally advanced, non-resectable cancers and the viability of C. noyvi-NT bacteria as a form of cancer therapy. Studies are being conducted to evaluate the most effective forms of bacteria and forms of cancer therapy to improve efficacy and to reduce variability.
Recombination cancer therapies
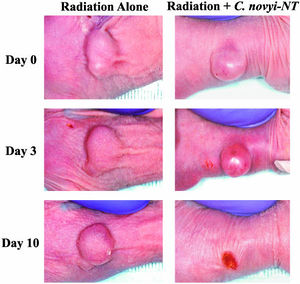
Reprinted from Chetan Bettegowda et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. PNAS 2003;100:15083-15088 ©2003 by National Academy of Sciences
In addition to studying the therapeutic effects of intratumoral injection of bacterial spores alone, researchers are also studying the effects of recombination cancer therapies. Bacteria may enhance the therapeutic effects of radiation by killing tumor regions resistant to radiation therapy when used in conjunction with radiation treatment.11
In one study, Bettegowda et. al (2006) compared the effects of spore injection alone and spore injection in combination with different types of radiation in varying amounts to determine the most effective form of treatment. Different forms of radiotherapy tested included External Beam Radiation, Brachytherapy, and RAIT (radioimmunotherapy).2 Other recombination cancer therapies are also currently being studied to improve their efficiencies in completely eliminating viable tumor cells.
Combination with External Beam Radiation
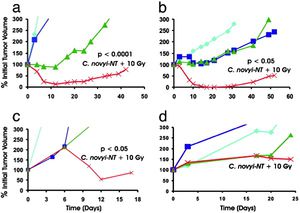
Spores were administered after the third dose of radiation. Tumor growth curves are color-coded: light blue-untreated control; dark blue-C. novyi-NT spores alone; green- radiation alone; red- radiation plus C. novyi-NT spores. In all cases across different cancer cell lines, the combined treatment (red) showed the highest marked reduction of tumor volume over time.
Reprinted from Chetan Bettegowda et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. PNAS 2003;100:15083-15088 ©2003 by National Academy of Sciences
To determine whether the combination of both C. novyi-NT and irradiation could eliminate both the well-vascularized and poorly vascularized regions of these tumors, tumor-bearing mice were treated with a protocol using five daily doses of external beam radiation, with spores administered between the third and fourth doses.2 The spores significantly enhanced the effect of radiation in most of these tests, and this combination therapy produced remarkable tumor shrinkage that was evident upon gross inspection and microscopically.2 There was complete or nearly complete lysis of all tumor cells independent of size, with tumors ranging from 50 to 1,000 mm3.2 11 Other tumor models were also tested to compare and determine the combined effects of external beam radiation and spore injection. Effectivity varied according to cancer cell line. C. novyi-NT spores significantly enhanced the effects of radiation in the human biliary cancer (HuCC-T1) and the mouse melanoma (B16) cell lines. In human colorectal cancer (HT-29) and lung cancer (Calu-3) cell lines, the spores did not enhance the effects of radiation.2 Although C. novyi-NT substantially improved the effects of external beam irradiation at safe doses of radiation, a small number of residual tumor cells eventually proliferated, resulting in tumor recurrence including in models most sensitive to the combination therapy.2
Combination with Brachytherapy
In another recombination therapy study, intravenous injection of C. noyvi spores was supplemented with brachytherapy, an advanced cancer treatment that delivers higher doses of radiation to more specific areas of the body through implanted radiation sources inside the body. A single dose of spores combined with brachytherapy resulted in 100% cure in mice, defined by 3 months without evidence of disease.2
Combination with RAIT
The effects of C. novyi-NT given in combination with RAIT (radioactive immunotherapy) have also been studied.2 RAIT is a form of radiotherapy in which radioactive antibodies are used to target tumors. RAIT is used to treat systemic disease and is dependent on the vascularization and oxygen delivery of radiolabeled compounds to tumor cells. As both vascularization and oxygenation are compromised in poorly vascularized and hypoxic tumors, C. novyi-NT was studied to see if it could enhance the effects of RAIT. The experiment found that the combination therapy was effective regardless of whether the spores were administered before or after injection of the labeled antibody. Two of 14 mice treated with both RAIT and spores combination therapy were cured after a follow-up period of 6 months. Mice treated with RAIT alone, on the other hand, showed excessive tumor growth.2
Clostridia continues to be under investigation for cancer therapy uses in humans. Although Clostridia is not yet completely efficient to sufficiently and quantitatively control tumor growth, recent combination treatments have improved in preclinical antitumor responses. Combination of clostridial spores administration with current cancer therapies reduce the probability of incomplete tumor cell death or tumor regrowth. Further studies are being done on C. noyvi recombination therapies. In addition, Clostridium species have also been engineered to produce proteins of interest in antitumor therapy.1
Other bacterial treatments
Ongoing studies are searching for the best combinations or recombinant cancer therapies using a bacterial-directed approach. Other anaerobic bacterial species under investigation for cancer therapy include Bifodobacterium, Streptococcus pyogenes, and Salmonella Typhimurium. Streptococcus pyogenes and Salmonella typhimurium have been used with some success in clinical trials.13 Phase 1 clinical trials of S. typhimurium in both dogs and human patients have demonstrated that safe administration and tumor specificity, with limited efficacy. Genetically modified strains of the bacterium such as incorporating cytosine deaminase, have been developed to enhance efficacy with S. typhimurium therapy.13
References
1. Barbé, S., Van Mellaert, L., & Anné, J. (2006). The use of clostridial spores for cancer treatment. Journal of applied microbiology, 101(3), 571-578.[1]
2. Bettegowda, C., Dang, L. H., Abrams, R., Huso, D. L., Dillehay, L., Cheong, I., ... & Zhou, S. (2003). Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proceedings of the National Academy of Sciences, 100(25), 15083-15088. [2]
3. Bettegowda, C., Huang, X., Lin, J., Cheong, I., Kohli, M., Szabo, S. A., ... & Zhou, S. (2006). The genome and transcriptomes of the anti-tumor agent Clostridium novyi-NT. Nature biotechnology, 24(12), 1573-1580. [3]
4. Cheong, I., Huang, X., Bettegowda, C., Diaz, L. A., Kinzler, K. W., Zhou, S., & Vogelstein, B. (2006). A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science, 314(5803), 1308-1311.[4]
5. Connell, H. C. (1935). The study and treatment of cancer by proteolytic enzymes: preliminary report. Canadian Medical Association journal, 33(4), 364.[5]
6. Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W., & Vogelstein, B. (2001). Combination bacteriolytic therapy for the treatment of experimental tumors. Proceedings of the National Academy of Sciences, 98(26), 15155-15160.[6]
7. Lemmon, M. J., Van Zijl, P., Fox, M. E., Mauchline, M. L., Giaccia, A. J., Minton, N. P., & Brown, J. M. (1997). Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene therapy, 4(8), 791-796.[7]
8. Liu, S. C., Minton, N. P., Giaccia, A. J., & Brown, J. M. (2002). Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene therapy, 9(4), 291-296.[8]
9. Malmgren, R. A., & Flanigan, C. C. (1955). Localization of the vegetative form of Clostridium tetani in mouse tumors following intravenous spore administration. Cancer research, 15(7), 473-478.[9]
10. McCarthy, E. F. (2006). The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. The Iowa orthopaedic journal, 26, 154.[10]
11. Minton, N. P. (2003). Clostridia in cancer therapy. Nature Reviews Microbiology, 1(3), 237-242.[11]
12. Patyar, S., Joshi, R., Byrav, D. S., Prakash, A., Medhi, B., & Das, B. K. (2010). Review Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci, 17(1), 21-30.[12]
13. Pawelek, J. M., Low, K. B., & Bermudes, D. (2003). Bacteria as tumour-targeting vectors. The lancet oncology, 4(9), 548-556.[13]
14. Plomp, M., McCaffery, J. M., Cheong, I., Huang, X., Bettegowda, C., Kinzler, K. W., ... & Malkin, A. J. (2007). Spore coat architecture of Clostridium novyi NT spores. Journal of bacteriology, 189(17), 6457-6468.[14]
15. Roberts, N. J., Zhang, L., Janku, F., Collins, A., Bai, R. Y., Staedtke, V., ... & Zhou, S. (2014). Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Science translational medicine, 6(249), 249ra111-249ra111. [15]
16. Ryan, R. M., Green, J., & Lewis, C. E. (2006). Use of bacteria in anti-cancer therapies. Bioessays, 28(1), 84-94.[16]
17. Thiele, E. H., Arison, R. N., & Boxer, G. E. (1964). Oncolysis by clostridia. III. Effects of clostridia and chemotherapeutic agents on rodent tumors. Cancer research, 24(2 Part 1), 222-233.[17]
18. Van Mellaert, L., Barbé, S., & Anné, J. (2006). Clostridium spores as anti-tumour agents. TRENDS in Microbiology, 14(4), 190[18]
Edited by Anh Tran, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2015.
