Cercopithecine herpesvirus I: Difference between revisions
ABoone6632 (talk | contribs) No edit summary |
ABoone6632 (talk | contribs) |
||
| Line 7: | Line 7: | ||
Due to the extremely dangerous nature of this virus and the rarity of human infection, little research has been conducted on Herpes B, especially since personal protective gear requirements have been updated and strengthened over recent decades in biomedical testing centers throughout the United States. Much of what is known about Herpes B, therefore, has been found through comparison with [http://en.wikipedia.org/wiki/Herpes_simplex_virus HSV1] and [http://en.wikipedia.org/wiki/Herpes_simplex_virus HSV2] (5). It's viral biology closely follows HSV1, and so does the way that the infection presents itself in its natural host, the macaque. However, when transferred to humans, the virus is fatal more often than not, and steps must be taken to ensure human safety. | Due to the extremely dangerous nature of this virus and the rarity of human infection, little research has been conducted on Herpes B, especially since personal protective gear requirements have been updated and strengthened over recent decades in biomedical testing centers throughout the United States. Much of what is known about Herpes B, therefore, has been found through comparison with [http://en.wikipedia.org/wiki/Herpes_simplex_virus HSV1] and [http://en.wikipedia.org/wiki/Herpes_simplex_virus HSV2] (5). It's viral biology closely follows HSV1, and so does the way that the infection presents itself in its natural host, the macaque. However, when transferred to humans, the virus is fatal more often than not, and steps must be taken to ensure human safety. | ||
==<b>The Virus<b>== | ==<b>The Virus</b>== | ||
====<br>< | ====<br><u>Viral biology</u>==== | ||
<br>The family of herpesviruses has been divided into three main categories: <i>Alphaherpesvirinae</i>, <i>Betaherpesvirinae</i>, and <i>Gammaherpesvirinae</i>. The three categories were originally separated based on biological and molecular properties of the viruses. The α-herpesviruses, of which Herpes B is a member, generally initiate infection in mucosal epithelial cells. They locally replicate, and later invade sensory neurons. It is here that they form a latent infection in ganglia near the area of inoculation. Most α-herpesviruses also replicate quickly compared to the other two categories. In species that are not their natural hosts, the α-herpesviruses can produce disease with neurological involvement (7). Because of these dangers regarding cross-species infections, the α-herpesvirus group is potentially a substantial problem in using nonhuman primates as biomedical models of human disease. Herpes B is the only one herpesvirus that has been identified so far that is a pathogenic for humans (5). It was also the first nonhuman primate herpesvirus to be identified (8). <br> | <br>The family of herpesviruses has been divided into three main categories: <i>Alphaherpesvirinae</i>, <i>Betaherpesvirinae</i>, and <i>Gammaherpesvirinae</i>. The three categories were originally separated based on biological and molecular properties of the viruses. The α-herpesviruses, of which Herpes B is a member, generally initiate infection in mucosal epithelial cells. They locally replicate, and later invade sensory neurons. It is here that they form a latent infection in ganglia near the area of inoculation. Most α-herpesviruses also replicate quickly compared to the other two categories. In species that are not their natural hosts, the α-herpesviruses can produce disease with neurological involvement (7). Because of these dangers regarding cross-species infections, the α-herpesvirus group is potentially a substantial problem in using nonhuman primates as biomedical models of human disease. Herpes B is the only one herpesvirus that has been identified so far that is a pathogenic for humans (5). It was also the first nonhuman primate herpesvirus to be identified (8). <br> | ||
Revision as of 16:34, 14 April 2015
Out of the 35 herpesviruses that have been identified in nonhuman primates, only Cercopithecine herpesvirus 1, also known as Herpes B or B virus, has been identified as pathogenic for humans (5). Because of the B virus’s prevalence in macaques and other biomedical research animals, study of the B virus is important for several reasons. These reasons include understanding how it functions and whether or not it skews the results of any kind of biomedical testing, its interspecial and intraspecial transmission abilities, and its potential harm both to its regular hosts and less common hosts, such as humans (7).
Due to the extremely dangerous nature of this virus and the rarity of human infection, little research has been conducted on Herpes B, especially since personal protective gear requirements have been updated and strengthened over recent decades in biomedical testing centers throughout the United States. Much of what is known about Herpes B, therefore, has been found through comparison with HSV1 and HSV2 (5). It's viral biology closely follows HSV1, and so does the way that the infection presents itself in its natural host, the macaque. However, when transferred to humans, the virus is fatal more often than not, and steps must be taken to ensure human safety.
The Virus
Viral biology
The family of herpesviruses has been divided into three main categories: Alphaherpesvirinae, Betaherpesvirinae, and Gammaherpesvirinae. The three categories were originally separated based on biological and molecular properties of the viruses. The α-herpesviruses, of which Herpes B is a member, generally initiate infection in mucosal epithelial cells. They locally replicate, and later invade sensory neurons. It is here that they form a latent infection in ganglia near the area of inoculation. Most α-herpesviruses also replicate quickly compared to the other two categories. In species that are not their natural hosts, the α-herpesviruses can produce disease with neurological involvement (7). Because of these dangers regarding cross-species infections, the α-herpesvirus group is potentially a substantial problem in using nonhuman primates as biomedical models of human disease. Herpes B is the only one herpesvirus that has been identified so far that is a pathogenic for humans (5). It was also the first nonhuman primate herpesvirus to be identified (8).
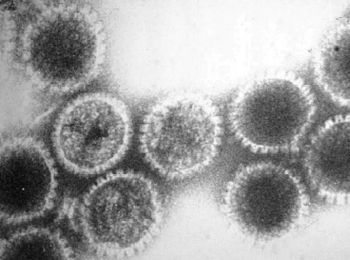
Herpes B is a large, double stranded DNA virus that has a structure typical to herpesviruses. This includes an amorphous tegument protein layer and lipid envelope studded with viral glycoproteins which surrounds the icosapentahedral capsid structure, which in turn surrounds electron dense core with viral DNA (5). The genome sequence of the standard vaccine for Herpes B has been sequenced by several laboratories. In addition, sequences for many single genes that came from field isolates of Herpes B have been found. The DNA genomes of simian α-herpesviruses are high in G+C content. Herpes B tops this list, with 74.5% of its content being cytosine and guanine (3). In addition, the genomes of simian viruses have been found to be very similar to the genomes of HSV1 and HSV2.
While other primate α-herpesviruses are Risk Group 2 pathogens and can be studied under Biosafety Level 2 containment, Herpes B is classified as a Risk Group 4 pathogen (3). This requires very high levels of Biosafety Level containment, and is partially responsible for the lack of research so far conducted on the Herpes B virus.
B Virus in Non-Human Primates
In macaque monkeys, who are the natural host for Herpes B, severe disease resulting from Herpes B infection does not commonly occur. In fact, infection of Herpes B in macaques causes symptoms that are similar to those caused by HSV-1 and HSV-2 in humans (5). However, Herpes B can still be dangerous in macaques with immunodeficiencies (1).
It has been found that most Herpes B infections in monkeys are acquired through routes other than venereal exposure, as is usually assumed in Herpes infections. The virus most commonly appears to be transmitted through biting and scratching (6). This method requires direct inoculation of virus by transferring of the infected monkey's saliva into the receiving monkey's bloodstream. This information is important when attempting to create Herpes B-free research colonies derived from infected groups, and illustrates that focusing on pubertal age or breeding opportunities will not solve the problem because it is not a sexual problem. Biting and scratching, while more common at a young age, can exist between animals both young and old and is a common form of light violence that monkeys may use to exert their dominance and maintain the social structure of the colony.
B Virus in Humans
First Documented Human Case
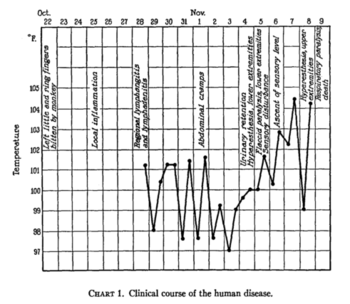
Sabin, A. B., & Wright, A. M. (1934). ACUTE ASCENDING MYELITIS FOLLOWING A MONKEY BITE, WITH THE ISOLATION OF A VIRUS CAPABLE OF REPRODUCING THE DISEASE. The Journal of experimental medicine, 59(2), 115-136.
On October 22nd, 1932, a Dr. W. B. was engaged in experimental work on poliomyelitis when he was bitten on the dorsum of the left ring and little fingers by a Macacus rhesus monkey who appeared normal. The superficial wounds were treated with iodine and alcohol. Three days later, however, Dr. B. was experiencing pain, redness, and slight swelling at the wound sites. By October 28th, he was admitted to the hospital with lymphangitis and a 101.4 degree fever. After a tetanus antitoxin injection had been administered and he appeared to improve in health, he obtained stomach cramps. By November 4th, he had hyperesthesia of the lower extremities and urinary retention. Over the next few days paralysis moved upwards from the lower extremities. Soon he had paresthesias in the upper extremities, a 104.8 degree fever, and he was put on a respirator for a diminishing respiratory rate. After a convulsion with laryngeal spasm, he lost consciousness and developed pulmonary edema. Despite medical support, he died on November 8th, just 18 days after receiving the bite (8).
This case was later found to be the first documented infection of Herpes B in a human. The virus was isolated from the brain, spinal cord, and spleen postmortem (7, 8).
Human Herpes B Infection
Since the original case in 1932, there have been a number of additional human infections. Though the infections are not common, they are well known in the biomedical research field because of their severity. Most human cases have been linked to bites or scratches from a macaque, but other cases have been traced back to needlestick injuries, contamination of open wounds with macaque cells in the laboratory, and the splashing of macaque bodily fluids such as urine into the eye (5).
When left untreated, infection in humans causes a 70-80% fatality rate, and those who do survive are left with serious neurologic deficits. Due to the increasing availability of antiviral drugs and immediate prophylactic treatment of those exposed to Herpes B, the survival rate is increasing.
Preventing Human Exposure
The main setting for human exposure to Herpes B is in the biomedical research field. Nonhuman primates are commonly used in biomedical research because they are genetically, anatomically, and physiologically similar to humans (5). In this field, humans often come in direct contact with macaques as well as their tissues and their fluids.
References
1. Bailey, C.C. & Miller, A.D. Ulcerative Cheilitis in a Rhesus Macaque.Veterinary Pathology 49, 412-415 (2012).
2. Cohen, J. I., Davenport, D. S., Stewart, J. A., Deitchman, S., Hilliard, J. K., & Chapman, L. E. (2002). Recommendations for prevention of and therapy for exposure to B virus (cercopithecine herpesvirus 1). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 35(10), 1191-1203.
3. Elmore, D. & Eberle, R. Monkey B virus (Cercopithecine herpesvirus 1).Comparative Medicine 58, 11-21 (2008).
4. Estep, R.D., Messaoudi, I. & Wong, S.W. Simian herpesviruses and their risk to humans. Vaccine 28, (2010).
5. Huff, J.L. & Barry, P.A. B-virus (Cercopithecine herpesvirus 1) infection in humans and macaques: Potential for zoonotic disease. Emerging Infectious Diseases 9, 246-250 (2003).
6. Weigler, B.J., Scinicariello, F. & Hilliard, J.K. Risk of venereal B virus (cercopithecine herpesvirus 1) transmission in rhesus monkeys using molecular epidemiology. The Journal of infectious diseases 171, 1139-1143 (1995).
7. Eberle, R. & Hilliard, J. The simian herpesviruses. Infectious agents and disease 4, 55-70 (1995).
8. Sabin, A. B., & Wright, A. M. (1934). ACUTE ASCENDING MYELITIS FOLLOWING A MONKEY BITE, WITH THE ISOLATION OF A VIRUS CAPABLE OF REPRODUCING THE DISEASE. The Journal of experimental medicine, 59(2), 115-136.
Edited by Alexis Boone, a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.

