Candidatus Prometheoarchaeum: Difference between revisions
No edit summary |
No edit summary |
||
| Line 44: | Line 44: | ||
[[File:Iivi1.gif|200px|thumb|left|alt text]] | [[File:Iivi1.gif|200px|thumb|left|alt text]] | ||
>[[File:Iivi2.jpg|200px|thumb|left|alt text]] | |||
Candidatus Prometheoarchaeum syntrophicum’ strain MK-D1 is an anaerobic, extremely slow-growing, small coccus (around 550 nm in diameter) that degrades amino acids through syntrophy. Microscopic observations showed that the cells are small cocci, ca. 300-750 nm in diameter (average 550 nm, n=15), and generally form aggregates surrounded with extracellular polysaccharide (EPS)-like materials. The cells also form unique membrane-based protrusions with a diameter of about 80–100 nm and various lengths (Aoki, M. et al 2014). Some protrusions remarkably display complex branching, unlike known archaeal protrusions (Marguet, E. et al 2013.) Dividing cells have less EPS-like materials and a ring-like structure around the middle of cells. | Candidatus Prometheoarchaeum syntrophicum’ strain MK-D1 is an anaerobic, extremely slow-growing, small coccus (around 550 nm in diameter) that degrades amino acids through syntrophy. Microscopic observations showed that the cells are small cocci, ca. 300-750 nm in diameter (average 550 nm, n=15), and generally form aggregates surrounded with extracellular polysaccharide (EPS)-like materials. The cells also form unique membrane-based protrusions with a diameter of about 80–100 nm and various lengths (Aoki, M. et al 2014). Some protrusions remarkably display complex branching, unlike known archaeal protrusions (Marguet, E. et al 2013.) Dividing cells have less EPS-like materials and a ring-like structure around the middle of cells. | ||
| Line 49: | Line 51: | ||
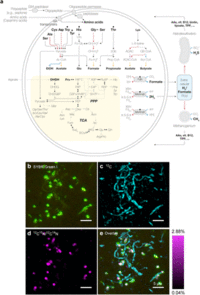
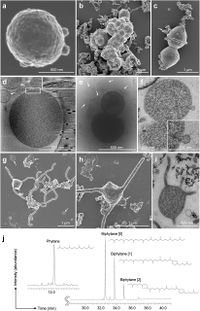
Cryo-electron and transmission electron microscopic observations revealed that the cells contain no visible organelle-like inclusions (Fig. 3d–f, Extended Data Fig. 2 and Supplementary Movies S1–S3). The cells produce membrane vesicles (MVs; 50–280 nm in diameter) (Fig. 3d–f and Extended Data Fig. 2) and chains of blebs (Fig. 3c and Extended Data Fig. 2e). The cells also form unique membrane-based protrusions with a diameter of about 80–100 nm and various lengths (Fig. 3g–i and Extended Data Fig. 2). Some protrusions remarkably display complex branching, unlike known archaeal protrusions18. These protrusions are especially abundant after late exponential growth phase. Lipid composition analysis of the MK-D1 and Methanogenium co-culture revealed typical archaeal signatures – a C20-phytane and C40-biphytanes (BPs) with 0–2 cyclopentane rings (Fig. 3j). Considering the lipid data obtained from a reference Methanogenium isolate (99.3% 16S rRNA gene identity; Supplementary Fig. S3), MK-D1 probably contains C20-phytane and C40-BPs with 0–2 rings. | Cryo-electron and transmission electron microscopic observations revealed that the cells contain no visible organelle-like inclusions (Fig. 3d–f, Extended Data Fig. 2 and Supplementary Movies S1–S3). The cells produce membrane vesicles (MVs; 50–280 nm in diameter) (Fig. 3d–f and Extended Data Fig. 2) and chains of blebs (Fig. 3c and Extended Data Fig. 2e). The cells also form unique membrane-based protrusions with a diameter of about 80–100 nm and various lengths (Fig. 3g–i and Extended Data Fig. 2). Some protrusions remarkably display complex branching, unlike known archaeal protrusions18. These protrusions are especially abundant after late exponential growth phase. Lipid composition analysis of the MK-D1 and Methanogenium co-culture revealed typical archaeal signatures – a C20-phytane and C40-biphytanes (BPs) with 0–2 cyclopentane rings (Fig. 3j). Considering the lipid data obtained from a reference Methanogenium isolate (99.3% 16S rRNA gene identity; Supplementary Fig. S3), MK-D1 probably contains C20-phytane and C40-BPs with 0–2 rings. | ||
Revision as of 22:49, 30 April 2020
Classification
Domain: Archaea
Kingdom: Proteoarchaeota
Superphylum: Asgard
Phylum: Lokiarchaeota
Genus: Candidatus
Species: Prometheoarchaeum syntrophicum "Imachi et al. 2020"
Strain: MK-D1
Use NCBI link to find]
Species
|
NCBI: Taxonomy |
Candidatus Prometheoarchaeum syntrophicum strain MK-D1
Description and Significance
Describe the appearance, habitat, etc. of the organism, and why you think it is important.
Starting from deep-sea sediments to a bioreactor-based “pre-enrichment” and a final seven-year in vitro enrichment Hiroyuki Imachi dubbed the isolated Lokiarchaeon “Candidatus Prometheoarchaeum syntrophicum strain MK-D1”. Current data suggest that eukaryotes may have risen from the archaeal lineage known as "Asgard archaea" (Spang A. et al. 2015). Although a resemblance of eukaryote-like genomic features have been discovered in these archaea, the evolutionary transition from archaea to eukaryotes remains uncertain due to the lack of cultured representatives and corresponding physiological insights. Given the proposed eukaryote-like intracellular complexities for Asgard archaea, the MK-D1 isolate has no visible organelle-like structure. Morphological features of Candidatus Prometheoarchaeum syntrophicum are of unique complexity; long and branching protrusions.
Genome Structure
Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence?
Cell Structure, Metabolism and Life Cycle
Interesting features of cell structure; how it gains energy; what important molecules it produces.
>
Candidatus Prometheoarchaeum syntrophicum’ strain MK-D1 is an anaerobic, extremely slow-growing, small coccus (around 550 nm in diameter) that degrades amino acids through syntrophy. Microscopic observations showed that the cells are small cocci, ca. 300-750 nm in diameter (average 550 nm, n=15), and generally form aggregates surrounded with extracellular polysaccharide (EPS)-like materials. The cells also form unique membrane-based protrusions with a diameter of about 80–100 nm and various lengths (Aoki, M. et al 2014). Some protrusions remarkably display complex branching, unlike known archaeal protrusions (Marguet, E. et al 2013.) Dividing cells have less EPS-like materials and a ring-like structure around the middle of cells.
Cryo-electron and transmission electron microscopic observations revealed that the cells contain no visible organelle-like inclusions (Fig. 3d–f, Extended Data Fig. 2 and Supplementary Movies S1–S3). The cells produce membrane vesicles (MVs; 50–280 nm in diameter) (Fig. 3d–f and Extended Data Fig. 2) and chains of blebs (Fig. 3c and Extended Data Fig. 2e). The cells also form unique membrane-based protrusions with a diameter of about 80–100 nm and various lengths (Fig. 3g–i and Extended Data Fig. 2). Some protrusions remarkably display complex branching, unlike known archaeal protrusions18. These protrusions are especially abundant after late exponential growth phase. Lipid composition analysis of the MK-D1 and Methanogenium co-culture revealed typical archaeal signatures – a C20-phytane and C40-biphytanes (BPs) with 0–2 cyclopentane rings (Fig. 3j). Considering the lipid data obtained from a reference Methanogenium isolate (99.3% 16S rRNA gene identity; Supplementary Fig. S3), MK-D1 probably contains C20-phytane and C40-BPs with 0–2 rings.
Ecology and Pathogenesis
Habitat; symbiosis; biogeochemical significance; contributions to environment.
If relevant, how does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
Based on cultivation and genomics, we propose an “Entangle-Engulf-Enslave (E3) model” for eukaryogenesis through archaea-alphaproteobacteria symbiosis mediated by the physical complexities and metabolic dependency of the hosting archaeon.
References
Imachi H, Nobu MK, Nakahara N, et al. Isolation of an archaeon at the prokaryote-eukaryote interface. Nature. 2020;577(7791):519‐525. doi:10.1038/s41586-019-1916-6
Spang, A. et al. Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 521, 173–179 (2015).
Aoki, M. et al. A long-term cultivation of an anaerobic methane-oxidizing microbial community from deep-sea methane-seep sediment using a continuous-flow bioreactor. PLoS ONE 9, e105356 (2014).
Marguet, E. et al. Membrane vesicles, nanopods and/or nanotubes produced by hyperthermophilic archaea of the genus Thermococcus. Biochem. Soc. Trans. 41, 436–442 (2013).
Author
Page authored by Jeremy Eugene Patrick, student of Prof. Jay Lennon at Indiana University.