Small Intestine: Difference between revisions
| Line 89: | Line 89: | ||
====Small Intestine Bacterial Overgrowth==== | ====Small Intestine Bacterial Overgrowth==== | ||
Small intestine bacterial overgrowth (SIBO), also known as small bowel bacterial overgrowth (SBBO), is a condition of the small intestine, defined as an increase in the number of bacteria in the upper gastrointestinal tract. It can be caused by any condition that interferes with the muscular activity in the small intestine that can allow bacteria to multiply. SIBO can result in villous atrophy and mucosal inflammation, altering the absorptive functions of the small intestine. The main cause of SIBO is thought to be mainly bacterial. The production of enterotoxins by facultative anaerobes injures the intestinal surface while aerobic bacteria produce enzymes and metabolic products that cause epithelial injury. Symptoms of SIBO result mainly from malabsorption, and these symptoms include combinations of cramping, diarrhea, dyspepsia, and weight loss. In addition, anemia can result from malabsorption caused by occult blood loss, vitamin B12 deficiency, or both. | |||
The diagnosis of SIBO usually starts with a clinical exam. The presence of 10^5 or more colony units of non-pharyngeal bacteria, usually coliforms, suggest SIBO. Screening tests, which include urine ladicans, serum D-lactic acid, and the glucose breath hydrogen test, are also used. The most common of these, the glucose breath hydrogen test, works as an increase in the hydrogen production in the breath after glucose consumption usually means that there is a significant small intestine bacterial fermentation of carbohydrates. | |||
Treatment is usually given when the symptoms become too severe, and they usually depend on the species of bacteria involved and the severity of the symptoms. Most treatments include the use of oral antibiotics, which reduce the number of bacteria in the intestinal tract. Surgery may be needed for anatomical anomalies such as diverticula, which are pouches in the colon that provide bacteria with a safe area in which to multiply. The most popular antibiotic used is metronidazole. Trimethoprim-sulfamethoxazole, aminoglycosides, spectrum penicillins, and cephlasporins are usually used against facultative anaerobes. If a patient has increased symptomatic activity, increased dosage may be administered. Sulfasalazine or corticosteroids may be used if there is an increased inflammatory response, however, dosage is based only on bacterial response. | |||
A new and upcoming treatment used to combat SIBO is the use of bacterial substitution, which utilizes probiotic bacteria to replace existing bacteria, since the probiotic bacteria can eliminate pathogenic bacterial infections. These probiotic bacteria have a beneficial effect on the small intestine. The probiotic bacteria commonly used are able to control the growth of pathogenic bacteria such as Salmonella typhimurium, Shigella sp, Clostridium difficile, Campylobacter jejuni, and E. coli, and in addition, are able to offer protection against Gardnerella vaginalitis, Bacteroides bivius, Candida albicans, and Chlamydia trachomatis. Lactic acid bacteria such as Lactobacillus GG and Lactobacillus plantarum 299Vare able to inhibit many gram-negative bacteria by removing toxic substances in the small intestine and stimulating the immune system. | |||
==Current Research and Discoveries== | ==Current Research and Discoveries== | ||
Revision as of 09:16, 29 August 2008
This article is concerned with the microbes residing in one particular niche: the small intestine of the digestive tract. Although most of the human flora (the term used for the bacteria living in the human body) is found in the colon, there are a good number of microbes found in the small intestine. The following article will discuss the different bacteria that contribute to normal functioning and diseased states of the small intestine, with a focus on that of humans.
Introduction
The human body is not only made up of human cells, but is also comprised of bacterial cells. In fact, microorganisms, or microbes, are so abundant that there are about ten times as many bacteria as there are human cells; while there are 100 trillion human cells, there are 1000 trillion bacteria cells [12]. It is estimated that there are 500 to 100,000 species of bacteria living in the human body [12]. These microbes reside on the skin and mucus surfaces of human tissue, but not within tissues[10]. They are mostly found on seven surfaces: the skin, eyes, mouth, nose, vagina, small intestine, and colon.
Most of the bacteria in the body have neither a beneficial or harmful effect on the human host, but remain inside because they can flourish there; these “normal” bacteria have been dubbed microbiota [11]. However, there are several that indeed benefit the human (a relationship of mutualism), while there are some bacteria that are harmful to the host (a parasitic relationship). Parasitic bacteria can cause many different types of disease, and are thus also considered pathogenic bacteria. As this article is looking at the small intestine, different microbial diseases of the small intestine will be reviewed. It is interesting to note that the growth of pathogenic bacteria can be controlled via means of competitive exclusion provided by the presence of useful bacteria provides [13]; this demonstrates the importance of studying the interaction between different microorganisms in a niche, which will be covered later in this article.
Description of the niche
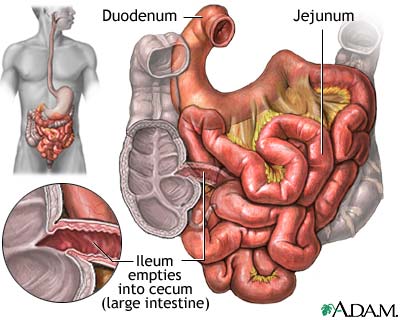
The small intestine is the site where most of the nutrient absorption occurs. After food has been broken down in the stomach by strong hydrochloric acid, the pyloric sphincter opens and food get pushed into small intestine by peristalsis. There are three major structural parts of the small intestine, duodenum, jejunum, and ileum. Throughout these sections, finger-liked structure called villi increase surface area that helps the absorption of nutrients. The concentration of bacterial activity in the small intestine is around 1 million per milliliter [1].
Duodenum
The duodenum is a hollowed tube that is situated between the stomach and the jejunum. The duodenum is the shortest section of the small intestine; it is 26cm long, on average. It is mostly responsible for digesting chyme, the food bolus that was created by the churning motion of the stomach. Since it doesn’t have the thick mucus lining like the stomach, the duodenum cannot tolerate the low pH introduced by the chyme. In order to neutralize the pH, the liver secrets bile and bicarbonate into the duodenum and brings the pH of duodenum up to around 5 and 6 range, a much more tolerable pH for protein and enzymes to function. Enzymes such as lipase, trypsin and chymotrypsin are also secreted into the duodenum to aid digestion. The bacterial density in this section of the small intestine reaches 101 to 103 CFU/mL [2].
Jejunum
The jejunum is 2.5m long, and is the site of absorption. The pH is 7-8 (slightly alkaline). The jejunum is situated right after the duodenum and it is about 10 feet long with pH between 7 and 8 [21]. Goblet cells are most numerous in jejunum, although they exist throughout small intestine. The primary functions of these cells are to secret mucus. Mucus provides protection against pH, stress, and microorganisms by trapping them [22]. As a result of this, the bacterial density rise to 104-107 CFU/mL.
Ileum
The ileum is the last section of the small intestine. Like the jejunum, it is also around 10 feet long and its pH lies between 7 and 8. The bacterial density here is also 104-107 CFU/mL. The Ileum is responsible for most of the food and liquid absorption, and the unabsorbed matter and waste products are passed into the large intestine. (muhealth) One unique feature of the ileum is the dominance of Peyer’s patch, a form of lymphoid tissue. The main function of these Peyer’s patches is to provide leukocytes, part of the immune system, to fight against foreign microorganisms [2].
Influence by adjacent communities
Stomach
The stomach is located directly above the small intestine, and it is separated from the duodenum by the pyloric sphincter. As mentioned above, the duodenum has a neutral pH, which is needed because inside the stomach, gastric juice is secreted in order to provide the acidic environment needed to convert the inactive pepsinogen to the active pepsin. As the chyme (the semi-digested foodstuff) enters the duodenum, bile is secreted by the liver through the bile duct, to neutralize the acidic pH from the stomach.
Colon (Large Intestine)
The colon is connected to the other end of the small intestine, at the ileum. It contains an incredibly large number of bacteria, at a concentration of 1011-1012 CFU/mL; the highest concentration found in any ecosystem [2].
Conditions under which the environment changes
Do any of the physical conditions change? Are there chemicals, other organisms, nutrients, etc. that might change the community of your niche.
Resident microbes
Which microbes are present?
The intestinal tract of human body has ten times as many microbial organisms than the rest of the body cells in the body [9]. There are up to 100,000 primarily aerobic organisms per milliliter found in the small intestine, and there are at least 500 bacterial species present inside the intestinal tract [9]. The bacterial environment of the jejunum, the middle section of the small intestine located after the duodenum, consists mainly of gram-positive Streptococci and Lactobacilli [6]. Research done on several healthy volunteers showed the presence of Streptococci, Lactobacilli, Staphylococci, and fungi in the intestinal tract [6]. In the ileocaecal valve, Bacteroides and coliform bacteria are the dominant bacteria present there, in addition to anaerobic Lactobacilli [6]. In the ileum, the last section of the small intestine, the bacterial presence is varied, due to the “backwash contamination of the colon” [6].
Protection against bacteria
Functionally similar to neutrophils, the most abundant form of white blood cell in human, paneth cells on the crypt in the lumen of the small intestine provide host defense against harmful bacteria by primarily secreting antibacterial molecules defensins, or alpha-defensins when exposed to both Gram positive and negative bacteria. Defensins kills the bacteria cells by disrupting their membrane function. Defensins peptide have hydrophobic and positively-charged regions that can interact with bacteria’s negatively phospholipids membrane by forming pores, leading to the weakening of the membrane and causing lysis of the bacterial cell. Since most bacteria have higher concentration of negatively charged membrane than normal vertebrae cell membrane, they interact well with defensins’ positively charged regions and sparing the normal vertebrae cells that are needed for normal metabolic functions. Paneth cells also secret lysozyme and phospholipase A2, which both have antimicrobial functions as well. With the addition of these two agents, paneth cells not only have the ability to kill bacteria, but even fungi and some enveloped viruses as well. [14]
The microbes of the small intestine carry out a number of functions.[2]
Protective functions
• The natural flora of the small intestine protect the host by taking up space inside the small intestine. Their presence in the small intestine prevents pathogens from obtaining a foothold in the niche.
• The natural flora of the small intestine provides competition for nutrients against pathogens, making it more difficult for pathogens to grow.
• The natural flora of the small intestine produces anti-bacterial products to eliminate competition such as pathogens.
Structural functions
• The natural flora in the small intestine makes up part of the intestinal barrier.
• The natural flora of the small intestine is critical in the natural development of the immune system.
Metabolic functions
• The natural flora of the small intestine protect the host by metabolizing carcinogens in dietary foods.
• The natural flora of the small intestine provide the host with synthesized vitamins, such as biotin and folate.
Microbial diseases of the small intestine
Peptic Ulcers from Helicobacter Pylori[3]
Crohn's Disease[4]
Salmonellosis[5]
Irritable Bowel Syndrome[6]
Necrotizing Entercolitis (NEC)[7]
Tropical Sprue
One of the infectious disorders of the small intestine is tropical sprue. Although there is no precise definition of this disorder as of yet, some scientists define it as “malabsorption of two or more test substances of people living in the tropics” [6]. Symptoms of tropical sprue usually include macrocytic anemia, or abnormally enlarged erythrocytes, due to a malabsorption of folate and vitamin B12. In early stages of this disease, the ileum and jejunum specifically are affected due to the malabsorption of xylose, glucose, fat, vitamin B12, and folate. After a duration of four months, the mucosa in the small intestine shows partial villous atrophy. Since the villi are the main components of the small intestine that is used for the absorption of nutrients, the absorptive qualities of the small intestine are limited in a patient with tropical sprue, leading to malabsorption of essential nutrients. Though there is no specific known cause of tropical sprue, there is little doubt that it is caused by a severe, acute gastrointestinal infection. No specific microorganism has been identified as the sole cause of tropical sprue, however. Patients with tropical sprue often have a colonization of coliform bacteria in the small intestine. Studies have shown that patients with tropical sprue in areas such as North India, Puerto Rico, Haiti, and in Europeans travelling in India contain coliform bacteria in the jejunum. The bacteria in the intestines of the European travelers included Alcaligenes faecaelis, Enterobacter aerogenes, and the hafnia species. Other patients were infested with Klebsiella pneumonia, E. coli, and Enterobacter cloacae. In one study in Vellore, India, coliform bacteria were present in 29 of 33 patients with tropical sprue. Additional studies in animals have shown that colonization of enterobacteria in the small intestine causes changes in mucosal structure of the villi. Several experiments have been conducted on rabbit jejunum. Coliform bacteria are usually aerobes or facultative anaerobes, and they contain toxins that increase fluid secretion in the intestines. Another experiment conducted on rabbit jejunum has shown that Klebsiella pneumonia, when entered into the jejunum, decreases xylose absorption while shortening and blunting the villi, inhibiting absorption. Similar results have been shown with a different strain of bacteria, Enterobacter cloacae, is also entered into the jejunum.
The standard treatment of tropical sprue is a dosage of tetracycline and folic acid for at least six months. This treatment fixes the mucosal structure of the villi of the small intestine, resolving malabsorption , resulting in patients having increased appetite and weight gain. If there is evidence of vitamin B12 malabsorption in the body during tropical sprue, a vitamin B12 replacement is added as a remedy. Though full recovery is expected, people who stay in the tropical areas where this disease is endemic, have a good chance of falling into a relapse. If this disease is untreated, death eventually occurs, with the victim dying extremely malnourished. However, tropical sprue can be fatal in extremely young or old victims, even with treatment.
Tropical sprue is mostly prevalent in tropical areas, however, not all of these areas have cases of tropical sprue. This may be due to dietary differences of the inhabitants in these regions. There has been a study linking the amount of long-chain unsaturated fatty acids consumed to the occurrence of tropical sprue in the population.
Small Intestine Bacterial Overgrowth
Small intestine bacterial overgrowth (SIBO), also known as small bowel bacterial overgrowth (SBBO), is a condition of the small intestine, defined as an increase in the number of bacteria in the upper gastrointestinal tract. It can be caused by any condition that interferes with the muscular activity in the small intestine that can allow bacteria to multiply. SIBO can result in villous atrophy and mucosal inflammation, altering the absorptive functions of the small intestine. The main cause of SIBO is thought to be mainly bacterial. The production of enterotoxins by facultative anaerobes injures the intestinal surface while aerobic bacteria produce enzymes and metabolic products that cause epithelial injury. Symptoms of SIBO result mainly from malabsorption, and these symptoms include combinations of cramping, diarrhea, dyspepsia, and weight loss. In addition, anemia can result from malabsorption caused by occult blood loss, vitamin B12 deficiency, or both.
The diagnosis of SIBO usually starts with a clinical exam. The presence of 10^5 or more colony units of non-pharyngeal bacteria, usually coliforms, suggest SIBO. Screening tests, which include urine ladicans, serum D-lactic acid, and the glucose breath hydrogen test, are also used. The most common of these, the glucose breath hydrogen test, works as an increase in the hydrogen production in the breath after glucose consumption usually means that there is a significant small intestine bacterial fermentation of carbohydrates.
Treatment is usually given when the symptoms become too severe, and they usually depend on the species of bacteria involved and the severity of the symptoms. Most treatments include the use of oral antibiotics, which reduce the number of bacteria in the intestinal tract. Surgery may be needed for anatomical anomalies such as diverticula, which are pouches in the colon that provide bacteria with a safe area in which to multiply. The most popular antibiotic used is metronidazole. Trimethoprim-sulfamethoxazole, aminoglycosides, spectrum penicillins, and cephlasporins are usually used against facultative anaerobes. If a patient has increased symptomatic activity, increased dosage may be administered. Sulfasalazine or corticosteroids may be used if there is an increased inflammatory response, however, dosage is based only on bacterial response.
A new and upcoming treatment used to combat SIBO is the use of bacterial substitution, which utilizes probiotic bacteria to replace existing bacteria, since the probiotic bacteria can eliminate pathogenic bacterial infections. These probiotic bacteria have a beneficial effect on the small intestine. The probiotic bacteria commonly used are able to control the growth of pathogenic bacteria such as Salmonella typhimurium, Shigella sp, Clostridium difficile, Campylobacter jejuni, and E. coli, and in addition, are able to offer protection against Gardnerella vaginalitis, Bacteroides bivius, Candida albicans, and Chlamydia trachomatis. Lactic acid bacteria such as Lactobacillus GG and Lactobacillus plantarum 299Vare able to inhibit many gram-negative bacteria by removing toxic substances in the small intestine and stimulating the immune system.
Current Research and Discoveries
1. Proliferation and Apoptosis of the Enterocyte is influenced by Bacteria
Prior studies have shown that the small intestines have undergone morphological changes, such as the crypt depth and villous height, after inoculating germ-free pigs with different types of bacteria. Two gnotobiotic experiments were performed where 16 piglets were allocated into 4 types of treatment groups: Germ-Free, monoassociation with Lactobacillus fermentum, Escherichia coli, or sow feces. The piglets were reared for 14 days of age where the intestinal tissue and enterocytes were collected each day for histology, gene expression, and protein analysis. Quantitative PCR was used to measure proliferating nuclear cell antigen and it was concluded that the E. coli and not the L. fermentum helped stimulate an increase apoptosis and cell proliferation. Thus, only by the death of the receptors and commensal bacteria were the enterocyte able to have a significant turnover.[3]
2. Commensal Bacteria increase invasion of intestinal epithelium
Researchers from Harvard Medical School have discovered that the bacteria in the small intestine may help promote the invasion of typhoid. The beneficial bacteria located in the small intestine produce a compound that assist in redistributing the protein on the cell’s surface, which resides on the lining of intestine. This triggers epithelial cell trafficking of a protein, therefore serving as a receptor for the pathogenic bacteria. As a result, the cells become more susceptible to infection of Salmonella enterica serovar Typhi.[4]
3. Diversity of Intestinal Bacterial in Maturing Chicken
Bacterial flora in the ilea and ceca of chickens are analyzed by studying 1,230 partial 16S rRNA gene sequences. Bacterial DNA was isolated using density gradient centrifugation. Studies show that the microbes that live in the ileum consist of: 68.5% Lactobacillus, 11% Clostridiaceae, 6.5% Streptococcus, and 6.5% Enterococcus. The large amount and variety of bacteria in the intestine make it difficult to distinguish the function of each one; however, overall that intestinal bacterial affect: nutrition, immune responses, pathogenesis of intestinal disease, and degradation of mucus. The bacterial community in birds is affected by diet, age, and antibiotics. Overall, results showed that as the bacterial community started out as a stable community in the ileum and cecum. However, as the chickens matured, the bacterial community becomes more complex and increasingly differs between the ileum and cecum, which shows that each region develops its own unique set of microbes.[16]
4. Regulation of Intestinal Angiogenesis by Microbes via Paneth Cells
Using three-dimensional imaging, researchers at the Washington University School of Medicine studied the impact of intestinal angiogenesis on the microbes in the small intestine of mice. Angiogenesis, the growth of new blood vessels, takes place in the complex network of blood vessels in small intestinal villi. However, the villi is also the site where gut microorganisms, or microbiota, live. This experiment studies the differences between the villi in germ-free mice and mice with Bacteroides thetaiotaomicron colonies during or after postnatal gut development – approximately a ten day period. Studies have shown that Bacteroides thetaiotaomicron is of the major microbes that reside in both mouse and human gut. This experiment focuses on Paneth cells, which defend the host against microbes by secreting antibacterial peptides that affect the microbes in the lumen, or interior of the blood vessels. The results show that microbes function in breaking down carbohydrate polymers by facilitating lumen breakdown of dietary macromolecules so that the host does not have to cleave the various linkages in their food themselves. Symbiosis is observed since the host is able to gain more nutrients, while microbes are provided a safe niche with a plentiful carbon source.[17]
5. Microbial Symbiosis prevents Intestinal Inflammatory Disease
Symbiosis between microbes and their human host can be observed by microbes preventing intestinal inflammatory disease. One of the main microbes this experiment focuses on is Bacteroides fragilis, which protects its human host against colitis, a chronic inflammation of the membrane lining the gastrointestinal tract, caused by Helicobacter hepaticus, a commensal bacterium. Although colitis is mainly affects the large intestine, in some rare cases it affects the ileum as well since it precedes the large intestine. In order for Bacteroides fragilis to be beneficial to its host, it must express polysaccharide A, or PSA, which affects the interleukin-10-producing CD4 T cells; otherwise Helicobacter hepaticus will continue to grow and cause inflammatory disease. It is shown that purified PSA can prevent gut pathology. This experiment gives purified PSA to mice and measures the changes of the inflammatory disease. Mice without Helicobacter hepaticus had a very mild colitis, while mice with Helicobacter hepaticus developed severe colitis. When purified PSA was given to the diseased mice, they were almost completely protected against the Helicobacter hepaticus, and the disease level was reduced to match those who did not develop colitis. Thus, Bacteroides fragilis is just one example of a symbiosis relationship where a single bacterial organism promotes human health; and suggests that there are others yet to be discovered.[18]
6. Effects of Dietary Fat Source and Antibiotics and Age on Bacterial in the Ileum of Chickens
PCR with denaturing gradient gel electrophoresis (DGGE) studies how different fat sources in the diets of broiler chickens affected the microbial community in the ileum of the chickens at different ages. DGGE involves molecular fingerprinting which separates polymerase chain reaction (PCR)-generated DNA products. This experiment differs from previous experiments, which only focused on the cecum, by examining the parts of the small intestine – the jejunum and ileum, which are largely responsible for nutrient absorption. The experiment focuses only on the ileum, but notes that the same micro flora exist in both sites. In addition, instead of studying the effects of dietary carbohydrate sources, it focuses on dietary fat sources by feeding the chickens with either soy oil or lard and tallow mix and an antibiotic supplement (avilamycin and salinomycin mix). Salinomycin antibiotic inhibits the growth of C. perfringens. The results confirmed that the microbial communities were affected by both factors: the chickens’‘ dietary fat source with antibiotics as well as the age of the chickens. As the chickens aged, there was an increase in streptococci Streptococcus alactolyticus, enterobacteria, and Clostridium perfringens. The different dietary fat sources primarily affected Lactobacilli Lactobacillus salivarius and Clostridium perfringens. Results show that soy oil has a higher solubility which help the dispersion of antibiotics in the small intestine; thus, indirectly affects the microbial communities that live there. Clostridium perfringens, which was affected by both age and dietary fat sources, also exhibited different strains in response to the factors.[19]
References
[1] Bentley-Hibbert, Dr. Stuart. Small Intestine [The small intestine is the portion of the digestive system most responsible for absorption of nutrients from food into the bloodstream. The pyloric sphincter governs the passage of partly digested food from the stomach into the duodenum. This short first]. Digital image. MedlinePlus Medical Encyclopedia. 25 Oct. 2006. ADAM. 26 Aug. 2008 <http://www.nlm.nih.gov/medlineplus/ency/imagepages/19221.htm>.
[2] O'Hara, Ann M., and Fergus Shanahan. "The gut flora as a forgotten organ." European Molecular Biology Organization 7 (2006): 688-93.
[3] Willing, B. P., and A.G. Van Kessel. "Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig." Journal of Animal Science 2527th ser. 85 (2007): 3256-266.
[4] Lyczak, J.B. "Commensal Bacteria Increase Invasion of Intestinal Epithelium by Salmonella enterica Serovar Typhi." INFECTION AND IMMUNITY 71 (2003): 6610-614.
[5] Baker, S.J. "Tropical Sprue." British Medical Bulletin 28 (1972): 87-91.
[6] Glynn, Judith. "Tropical sprue--its aetiology and pathogenesis." Journal of the Royal Society of Medicine 79 (1986): 599-606.
[7] Westgaard, Henrik. "Tropical Sprue." Current Treatment Options in Gastroenterology 7 (2004): 7-11.
[8] Lee, Dennis, and Jay W. Marks. "Small Intestine Bacterial Overgrowth." MedicineNet Inc. <http://www.medicinenet.com/small_intestinal_bacterial_overgrowth/article.htm#tocb>.
[9] Vanderhoof, Jon A., Rosemary J. Young, Nancy Murray, and Stuart S. Kaufman. "Treatment Strategies for Small Bowel Bacterial Overgrowth in Short Bowel Syndrome." Journal of Pediatric Gastroenterology & Nutrition 27 (1998): 155-60.
JANET'S REFERENCES:
[10]http://www.textbookofbacteriology.net/normalflora.html
[11]Samuel Baron MD; Charles Patrick. Davis (1996). "Bacteriology": Chapter 6. Normal Flora. University of Texas Medical Branch at Galveston.
[12]Sears CL (2005 Oct;11). "A dynamic partnership: celebrating our gut flora". Anaerobe (5):247-51: 247–251. Academic Press.
[13]Salminen S, Gueimonde M, Isolauri E (2005). "Probiotics that modify disease risk". J Nutr 135 (5): 1294 – 8. PMID 15867327.
[14]Wilson CL, Ouellette AJ, Satchell DP, etc: Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286:113-117, 1999.
[15]Nagi/Babiuk, A. M. "Preparation, purification and characterization of bovine Peyer's patch leukocytes." PudMed Central. Apr. 1988. Canadian Veterinary Medical Association. 29 Aug. 2008 <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1255435>.
[16]Lu, Jiangrang, Umelaalim Idris, Barry Harmon, Charles Hofacre, John J. Maurer, and Margie D. Lee. ‘‘Diversity and Succession of the Intestinal Bacterial Community of the Maturing Broiler Chicken.’‘ Applied and Environmental Microbiology 11th ser. 69 (2003): 6816-824.
[17]Stappenbeck, Thaddeus S., Lora V. Hooper, and Jeffrey I. Gordon. ‘‘Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells.’‘ Proceedings of the National Academy of Sciences 99 (2002): 15450-5455.
[18]Mazmanian, Sarkis K., June L. Round, and Dennis L. Kasper. ‘‘A microbial symbiosis factor prevents intestinal inflammatory disease.’‘ Nature 453 (2008): 620-25.
[19]Knarreborg, Ane, Mary Alice Simon, Ricarda M. Engberg, Bent Borg Jensen, and Gerald W. Tannock. ‘‘Effects of Dietary Fat Source and Subtherapeutic Levels of Antibiotic on the Bacterial Community in the Ileum of Broiler Chickens at Various Ages.’‘ Applied and Environmental Microbiology 68 (2002): 5918-924.
[20]Elmes/Stanton/Howells/Lowes. "Relation between the mucosal flora and Paneth cell population of human jejunum and ileum." PudMed Central. Nov. 1984. BMJ Group. 29 Aug. 2008 <http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=498996&pageindex=3#page>.
[21]"Anatomy and Function of the Gastrointestinal Tract." University of Missouri Health Care. University of Missouri. 29 Aug. 2008 <http://www.muhealth.org/weightlosssurgery/anatomy.shtml>.
[22]Gartner & Hiatt. "Jejunum." Histology Lab. University of Medicine and Dentistry of New Jersey. 29 Aug. 2008 <http://www3.umdnj.edu/histsweb/lab20/lab20jejunum.html>.
Edited by [Ellis Chiu, Janet Kwok, Kevin Lee, Susan Lee, Martin Tang, Adnan Vahora], students of Rachel Larsen