Bacterial nucleation in pseudomonas syringae: Difference between revisions
OconnorRyan (talk | contribs) No edit summary |
OconnorRyan (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
=Introduction= | =Introduction= | ||
Bacterial ice nucleation has been an area of study which began around the 1980s. Upon isolation of frost-damaged plant surface bacteria, species of <i>Pseudomonas</i> were found in high concentrations. From this it was thought that these bacteria maybe implicated with damage to plants in cold temperatures. Further analysis on particular species <i>Pseudomonas syringae</i> showed that there were surface proteins embedded in the membrane which act as nucleation sites. These nucleation sites allowed water molecules to become particularly aligned to promote freezing. Similar to a catalyst, these proteins were found to allow water to freeze at higher temperatures than in situations with the proteins absent. With the new technology of genetic manipulation, bacteria which had a mutation in this particular protein resulted in a lower freezing temperature with colonization on the surface of plants. | |||
This topic has many implications with the current society and its food crisis with starving countries. The weather is a massive determinant in crop harvests around the world. The fight against frost seems like a helpless battle. When plants are exposed to below-freezing temperatures ice crystals can form, causing many growth implications and tissue damage. On crops harboring the epiphyte <i>Pseudomonas syringae</i>, temperatures at which freezing occur usually range from 0-5°C (15). Commercial preparation of a mixture of naturally occurring Ice- strains is now available for control of frost injury on some crops (16). Current studies of bacterial ice nucleation are trying to link the weather to microbes in the upper atmosphere. As aerosol particles, bacterial cells can act as cloud condensation nuclei to form cloud droplets. There is a great deal of future study in these areas, as the weather affects all of earth’s surface inhabitants. | |||
=Pseudomonas syringae= | =Pseudomonas syringae= | ||
| Line 5: | Line 10: | ||
==Species== | ==Species== | ||
As a Gram-negative, rod-shaped, obligate aerobic bacterium, Pseudomonas syringae is one of 78 species that has been described in the Pseudomonas genus ( | As a Gram-negative, rod-shaped, obligate aerobic bacterium, <i>Pseudomonas syringae</i> is one of 78 species that has been described in the <i>Pseudomonas</i> genus (5). Characterized as an epiphyte, it grows supported non-parasitically by plants where it derives its nutrients and water from floating dust, rain, etc (10). This species can be found on tomatoes and beans to rice and tobacco, and is responsible for more surface frost damage to plants than any other mineral or organism (7). Its affiliation as a plant pathogen which causes disease in a large variety of plants makes this particular bacterium important in the field of food and biomass production, and is an important focus for the Department of Energy (1). <i>P. syringae</i> is a very stress-tolerant organism, and is the focus of many studies of stress-tolerant gene expression. Kurz <i>et al.</i>, (2010) used biochemical approaches to address water stress tolerance in <i>P. syringae</i>. They showed that different osmolytes differentially contribute to water stress tolerance and interact at the level of transcription. | ||
Different species of these flagellated, motile bacteria infect leaves and tissues of a wide range of hosts, but can be specific to a particular location of an individual. For example, Pseudomonas syringae pv. aesculi is able to infect vascular tissue to cause cankers in European horse chestnut in northwest Europe, but does not infect leaves, buds, or flowers on the same individual (Picture 2) ( | Different species of these flagellated, motile bacteria infect leaves and tissues of a wide range of hosts, but can be specific to a particular location of an individual. For example, <i>Pseudomonas syringae</i> pv. <i>aesculi</i> is able to infect vascular tissue to cause cankers in European horse chestnut in northwest Europe, but does not infect leaves, buds, or flowers on the same individual (Picture 2) (2). Some have also identified genes which are only expressed when the bacterium is on plants, which represent a ‘hidden genome’ not very well studied due to the absence in culture (1). Although there is variation with each pathovar, this bacterium grows optimally in cool, wet conditions from 15-25°C. It is proposed by Hirano <i>et al</i>. (1987) that momentum from falling raindrops is responsible for initiation of rapid growth and the forming of massive colonies on host surfaces (8). This has been seen as a method of how these bacterial cells are able to spread infection. This can also however, prevent infection by washing off bacterial cells from yet-infected surfaces. | ||
[[Image:Pseudosmonas_syringae_SEM.jpg|thumb|400px|right|[Image 1] | [[Image:Pseudosmonas_syringae_SEM.jpg|thumb|400px|right|[Image 1] | ||
| Line 17: | Line 22: | ||
==Importance== | ==Importance== | ||
This particular species of Pseudomonas has been the subject of a large array of studies over the past 35 years with its involvement in crop freezing. The majority of frost-sensitive plants usually suffer from damage between -2 and - | This particular species of <i>Pseudomonas</i> has been the subject of a large array of studies over the past 35 years with its involvement in crop freezing. The majority of frost-sensitive plants usually suffer from damage between -2 and -5<sup>o</sup>C (11, 12). When water gets this cold, water turns into ice in both inter- and intracellular ways, causing frost damage. <i>P. syringae</i> express a particular type of surface protein, ice-nucleation protein (INP), which increases temperatures to which water is able to freeze. In the absence of sites capable of ice nucleation, the cold water can supercool and freezing will not occur until the temperature is low enough for the most active ice nucleus available is able to catalyze crystallization of supercooled water (freezing)(4). Thus, supercooling instead of ice-nucleation could be a mechanism of frost protection. | ||
P. syringae is a species of bacteria very important to present-day society, particularly with the ever-increasing population of planet earth. Chronic hunger affects 820 million people worldwide, killing 25,000 people every day, one child every eight seconds (13). Large quantities of crops are lost every year are lost due to frost such as in the state of Florida, where 40% of the world’s orange juice supply is grown (14). A large area of interest with Pseudomonas syringae focuses on genetically engineering these bacteria with ice-nucleation-minus proteins. Without these proteins it is thought the temperatures tolerable by plants may be decreased. | <i>P. syringae</i> is a species of bacteria very important to present-day society, particularly with the ever-increasing population of planet earth. Chronic hunger affects 820 million people worldwide, killing 25,000 people every day, one child every eight seconds (13). Large quantities of crops are lost every year are lost due to frost such as in the state of Florida, where 40% of the world’s orange juice supply is grown (14). A large area of interest with <i>Pseudomonas syringae</i> focuses on genetically engineering these bacteria with ice-nucleation-minus proteins. Without these proteins it is thought the temperatures tolerable by plants may be decreased. | ||
=Ice Nucleation Active (INA) Proteins= | =Ice Nucleation Active (INA) Proteins= | ||
| Line 25: | Line 30: | ||
==Description== | ==Description== | ||
All proteins interact with water, but there are two classes in particular which have a function relating to ice: antifreeze proteins (AFPs) and ice-nucleation proteins (INPs). AFPs have particular structures known to inhibit formation of ice crystals, while INPs do just the opposite. INPs can be seen affiliated with the production of artificial snow ( | All proteins interact with water, but there are two classes in particular which have a function relating to ice: antifreeze proteins (AFPs) and ice-nucleation proteins (INPs). AFPs have particular structures known to inhibit formation of ice crystals, while INPs do just the opposite. INPs can be seen affiliated with the production of artificial snow (8). Pure water can be supercooled to -40<sup>o</sup>C in the absence of a heteronucleus (9). INPs are able to promote ice formation by raising the nucleation temperature, and in vitro this temperature can range from -14 to -2<sup>o</sup>C depending on the number of proteins that cluster together. | ||
==Structure== | ==Structure== | ||
Graether and Jia (2001) attempted to present a model of INP from Pseudomonas syringae based on comparison with two newly determined insect AFP structures. They analyzed the INP sequence of ~60 16-residue repeats similar to a different model organism, and proposed a 16-residue loop for P. syringae (Picture 3). Their result suggested that insect AFPs and bacterial INPs may have a similar B-helical structure, even though they have opposite effects on water molecules. | Graether and Jia (2001) attempted to present a model of INP from <i>Pseudomonas syringae</i> based on comparison with two newly determined insect AFP structures. They analyzed the INP sequence of ~60 16-residue repeats similar to a different model organism, and proposed a 16-residue loop for <i>P. syringae</i> (Picture 3). Their result suggested that insect AFPs and bacterial INPs may have a similar B-helical structure, even though they have opposite effects on water molecules. | ||
[[Image:Ice_Nucleating_Protein.png|thumb|400px|center|[Image 3] <br><i>Pseudomonas syringae</i> Cross section of modeled INP and a B-helical protein showing a wire frame representation of one loop. Cross section after 100 steps of energy minimization. Source: Graether and Jia, (2001).]] | [[Image:Ice_Nucleating_Protein.png|thumb|400px|center|[Image 3] <br><i>Pseudomonas syringae</i> Cross section of modeled INP and a B-helical protein showing a wire frame representation of one loop. Cross section after 100 steps of energy minimization. Source: Graether and Jia, (2001).]] | ||
| Line 35: | Line 40: | ||
==Bacterial Effects== | ==Bacterial Effects== | ||
Beginning more than 25 years ago, scientists began noticing that concentrations of bacteria residing on leaf surfaces were correlated with the temperatures at which freezing occurred in plant tissues. A study by Lindow et al., (1982) concluded that higher concentrations of P. syringae on leaf surfaces were associated with warmer temperatures of freezing (Figure 1). They also showed that freezing temperatures were much lower without the presence of the bacteria (Figure 2). These results led researchers to conclude that bacteria on the surfaces of leaves play a major role in plant freezing. | Beginning more than 25 years ago, scientists began noticing that concentrations of bacteria residing on leaf surfaces were correlated with the temperatures at which freezing occurred in plant tissues. A study by Lindow <i>et al.</i>, (1982) concluded that higher concentrations of <i>P. syringae</i> on leaf surfaces were associated with warmer temperatures of freezing (Figure 1). As concentrations of ice-nucleating active cells in water droplets increased, higher temperatures of freezing were seen. At concentrations of 3.5 x 10<sup>3</sup> <i>Pseudomonas syringae</i> cells/ml, less than half water droplets were still unfrozen at -9<sup>o</sup>C. They also showed that freezing temperatures were much lower without the presence of the bacteria on the surface of plant tissues (Figure 2). When concentrations of <i>P. syringae</i> were sprayed onto corn leaf disks half of the disks were frozen by temperatures of -3<sup>o</sup>C, whereas when sterilized water was used, half disks were unfrozen until -11<sup>o</sup>C. These results led researchers to conclude that bacteria on the surfaces of leaves play a major role in plant freezing. | ||
[[Image:Lindow et al.jpg|thumb|400px|right|[Figure 1] <br> Proportions of frozen droplets with respect to temperature at <i>Pseudomonas syringae</i> concentrations of 10<sup>7</sup>, 10<sup>6</sup>, 10<sup>5</sup>, 3.5<sup>4</sup>, and 3.5<sup>3</sup>. Cells were grown on nutrient agar and suspended in sterile water at appropriate concentrations. Source: Lindow <i>et al.</i>, 1982.]] | [[Image:Lindow et al.jpg|thumb|400px|right|[Figure 1] <br> Proportions of frozen droplets with respect to temperature at <i>Pseudomonas syringae</i> concentrations of 10<sup>7</sup>, 10<sup>6</sup>, 10<sup>5</sup>, 3.5<sup>4</sup>, and 3.5<sup>3</sup>. Cells were grown on nutrient agar and suspended in sterile water at appropriate concentrations. Source: Lindow <i>et al.</i>, 1982.]] | ||
[[Image:Lindow_et_al_sterile.jpg|thumb|400px|left|[Figure 2] <br> Ice nucleation activity of corn leaf disks from plants with and without leaf surface populations of <i>Pseudomonas syringae</i>. Plants were sprayed with suspensions of 2 x 10<sup>8</sup> cells/ml. Source: Lindow <i>et al.</i>, 1982.]] | [[Image:Lindow_et_al_sterile.jpg|thumb|400px|left|[Figure 2] <br> Ice nucleation activity of corn leaf disks from plants with and without leaf surface populations of <i>Pseudomonas syringae</i>. Plants were sprayed with suspensions of 2 x 10<sup>8</sup> cells/ml. Source: Lindow <i>et al.</i>, 1982.]] | ||
==Problems== | |||
In mutant lines of <i>P. syringae</i> which are ice-nucleation inactive (Ice<sup>-</sup>) have been genetically engineered and used in a variety of studies (22). Competition for limiting resources such as space and nutrients on leaf surfaces was suggested as a mechanism by which Ice<sup>-</sup> bacteria may prevent buildup of incoming Ice<sup>+</sup> strains. With the use of potato plants, reduced population sizes of Ice<sup>+</sup> bacteria treated with Ice<sup>-</sup> strains correlated with reduced amounts of frost injury during a natural radiative forest event. Thus, biologists are concerned that an introduction of bacteria with genetic mutation on the INA proteins might be detrimental to the survival of the original species. | |||
<br> | <br> | ||
| Line 92: | Line 101: | ||
The use of ice-nucleating bacteria has already been seen in uses for creating fake snow to protection of crops from frost during growing seasons. On a larger scale, IN bacteria has begun to be studied in the atmosphere. Mohler, <i>et al</i>. (2008) conducted a study investigating five different bacterial species in the range of -5 to -15<sup>o</sup>C and their correlations to condensation. <i>Pseudomonas syringae</i> resides on plant surfaces, where it is emitted into the atmosphere (17). Ice nucleation active strains have been detected in rain and snow, as well as in the atmosphere (18, 19), and suggests that they may be disseminated through the water cycle. Bacterial ice nucleation is an important area of study in the initiation of precipitation, and with particular species nucleation can occur at a range of temperatures. In this study, bacterial cells were suspended in the aerosol phase using the Aerosol Interaction and Dynamics in the Atmosphere facility in Germany. Growing water droplets and ice particles were sensitively detected using in situ light scattering and depolarization setup SIMONE, which measures the depolarization of polarized light at a particular wavelength scattered from particles in the center of the large, cold cloud chamber. Infrared extinction spectrometers were also used to characterize droplet and ice clouds. | The use of ice-nucleating bacteria has already been seen in uses for creating fake snow to protection of crops from frost during growing seasons. On a larger scale, IN bacteria has begun to be studied in the atmosphere. Mohler, <i>et al</i>. (2008) conducted a study investigating five different bacterial species in the range of -5 to -15<sup>o</sup>C and their correlations to condensation. <i>Pseudomonas syringae</i> resides on plant surfaces, where it is emitted into the atmosphere (17). Ice nucleation active strains have been detected in rain and snow, as well as in the atmosphere (18, 19), and suggests that they may be disseminated through the water cycle. Bacterial ice nucleation is an important area of study in the initiation of precipitation, and with particular species nucleation can occur at a range of temperatures. In this study, bacterial cells were suspended in the aerosol phase using the Aerosol Interaction and Dynamics in the Atmosphere facility in Germany. Growing water droplets and ice particles were sensitively detected using in situ light scattering and depolarization setup SIMONE, which measures the depolarization of polarized light at a particular wavelength scattered from particles in the center of the large, cold cloud chamber. Infrared extinction spectrometers were also used to characterize droplet and ice clouds. | ||
As a result, the IN active cell fraction was calculated from the ratio of ice particle number concentration to number of total cells. The bacteria investigated are mainly IN active between -7 and -11<sup>o</sup>C with an IN active fraction of the order of 10<sup>-4</sup>. This means that in similar conditions to what is present in the atmosphere, IN active bacteria are able to form ice crystals. From this data, future studies should test at what concentrations of IN active bacterial cells the initiation of precipitation occurs through the ice phase. More studies are needed to measure distribution, sources, and bacterial cell concentration in the troposphere, as well as characterization of IN active cells extracted from cloud and rain water. | |||
==Foods== | ==Foods== | ||
| Line 114: | Line 123: | ||
[4] Lindow, S.E., D.C. Arny, and C.D. Upper. 1982. Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiology 70: 1084-1089. | [4] Lindow, S.E., D.C. Arny, and C.D. Upper. 1982. Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiology 70: 1084-1089. | ||
[5] Anzai, Y., H. Kim, J. Park, H. Wakabayashi, and H. Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. International Journal of Systematic and Evolutionary Microbiology 50:1563-1589. | |||
[6] Kurz, M., A.Y. Burch, B. Seip, S.E. Lindow, and H. Gross. 2010. Genome-driven investigation of compatible solute biosynthesis pathways of Pseudomonas syringae pv. Sytingae and their contribution to water stress tolerance. Applied and Environmental Microbiology 76(16): 5452-5462. | |||
[7] http://pseudomonas-syringae.org/ | |||
[8] Margaritis, A., and A. S. Bassi. 1991. Principles and biotechnological applications of bacterial ice nucleation. Critical Reviews Biotechnology 11: 277–295. | |||
[9] Hobbs, P. V. 1974. Ice Physics. Clarendon Press, Oxford. | |||
[10] Dulla, G.F.J., K.V. Krasileva, and S.E. Lindow. 2010. Inference of quorum sensing in Pseudomonas syringae by bacterial epiphytes that limit iron availability. Environmental Microbiology 12(6): 1762-1774. | |||
[11] Burke, M.J., L.V. Gusta, H.A. Quamme, C.J. Weiser, and P.H. Li. 1976. Freezing and injury to plants. Annual Reviews of Plant Physiology 27: 507-528. | |||
[12] Levitt, J. 1972. Responses of plants to environmental stresses. Academic Press, New York. | |||
[13] Stanford, Claire. “World Hunger.” The Reference Shelf, 79(5). The H.W. Wilson Company, 2007. | |||
[14] Rottman, karl. “Freeze may take heavy toll on Forida citrus.” CNN US, January 11, 2010. Accessed from http://www.cnn.com/2010/US/weather/01/11/cold.weather/index.html?iref=allsearch. | |||
[15] Hirano, S.S., and C.D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae – a pathogen, ice nucleus, and epiphyte. American Society for Microbiology 64(3): 624-653. | |||
[16] Lindow, S.E. 1995. Control of epiphytic ice nucleation-active bacteria for management of plant frost injury, p. 239-256. In R.E. Lee, G.J. Warren, and L.V. Gusta, Biological ice nucleation and its applications. American Phytopathological Society, St. Paul, Minnesota. | |||
[17] Lindemann, J., H.A. Constantinidou, W.R. Barchet, and C.D. Upper. 1982. Plants as a source of airborne bacteria, including ice nucleation active bacteria. Applications in Environmental Microbiology 44: 1059-1063. | |||
[18] Morris, C.E., D.C. Sands, B.A. Vinatzer, C. Glaux, C. Guilbaud, A. Buffiere, S. Yan, H. Dominguez, and B.M. Thompson. 2008. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. The ISME Journal 2: 321-334. | |||
[19] Amato, P., M. Parazols, M. Sancelme, P. Laj, G. Mailhot, and A.M. Delort. 2007. Microorganisms isolated from the water phase of trophospheric clouds at the Puy de Dome: major groups and growth abilities at low temperatures. FEMS Microbiological Ecology 59: 242-254. | |||
[20] Mohler, O., D.G. Gerrgakopoulos, C.E. Morris, S. Benz, V. Ebert, S> Hunsmann, H. Saathoff, M. Schnaiter. 2008. Heterogeneous ice nucleation activity of bacteria: new laboratory experiments at simulated cloud conditions. Biogeosciences 5: 1425-1435. | |||
[21] Ryder, J.M., and T.C. Lee. 1988. Paper presented at the 1988 Annual Meeting of Institute of Food Technologists, New Orleans, Louisiana. Book of Abstracts. Pp151. Institute of Food Technologists, Chicago, Illinois. | |||
[22] Wilson, M., and S.E. Lindow. 1994. Ecological similarity and coexistence of epiphytic ice-nucleation (Ice+) Pseudomonas syringae strains and a non-ice-nucleating (Ice-) biological control agent. Applications of Environmental Microbiology 60: 3128-3137. | |||
Revision as of 04:01, 26 April 2011
Introduction
Bacterial ice nucleation has been an area of study which began around the 1980s. Upon isolation of frost-damaged plant surface bacteria, species of Pseudomonas were found in high concentrations. From this it was thought that these bacteria maybe implicated with damage to plants in cold temperatures. Further analysis on particular species Pseudomonas syringae showed that there were surface proteins embedded in the membrane which act as nucleation sites. These nucleation sites allowed water molecules to become particularly aligned to promote freezing. Similar to a catalyst, these proteins were found to allow water to freeze at higher temperatures than in situations with the proteins absent. With the new technology of genetic manipulation, bacteria which had a mutation in this particular protein resulted in a lower freezing temperature with colonization on the surface of plants.
This topic has many implications with the current society and its food crisis with starving countries. The weather is a massive determinant in crop harvests around the world. The fight against frost seems like a helpless battle. When plants are exposed to below-freezing temperatures ice crystals can form, causing many growth implications and tissue damage. On crops harboring the epiphyte Pseudomonas syringae, temperatures at which freezing occur usually range from 0-5°C (15). Commercial preparation of a mixture of naturally occurring Ice- strains is now available for control of frost injury on some crops (16). Current studies of bacterial ice nucleation are trying to link the weather to microbes in the upper atmosphere. As aerosol particles, bacterial cells can act as cloud condensation nuclei to form cloud droplets. There is a great deal of future study in these areas, as the weather affects all of earth’s surface inhabitants.
Pseudomonas syringae
Species
As a Gram-negative, rod-shaped, obligate aerobic bacterium, Pseudomonas syringae is one of 78 species that has been described in the Pseudomonas genus (5). Characterized as an epiphyte, it grows supported non-parasitically by plants where it derives its nutrients and water from floating dust, rain, etc (10). This species can be found on tomatoes and beans to rice and tobacco, and is responsible for more surface frost damage to plants than any other mineral or organism (7). Its affiliation as a plant pathogen which causes disease in a large variety of plants makes this particular bacterium important in the field of food and biomass production, and is an important focus for the Department of Energy (1). P. syringae is a very stress-tolerant organism, and is the focus of many studies of stress-tolerant gene expression. Kurz et al., (2010) used biochemical approaches to address water stress tolerance in P. syringae. They showed that different osmolytes differentially contribute to water stress tolerance and interact at the level of transcription.
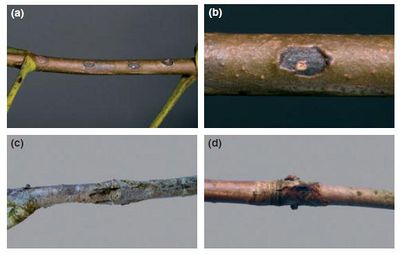
Different species of these flagellated, motile bacteria infect leaves and tissues of a wide range of hosts, but can be specific to a particular location of an individual. For example, Pseudomonas syringae pv. aesculi is able to infect vascular tissue to cause cankers in European horse chestnut in northwest Europe, but does not infect leaves, buds, or flowers on the same individual (Picture 2) (2). Some have also identified genes which are only expressed when the bacterium is on plants, which represent a ‘hidden genome’ not very well studied due to the absence in culture (1). Although there is variation with each pathovar, this bacterium grows optimally in cool, wet conditions from 15-25°C. It is proposed by Hirano et al. (1987) that momentum from falling raindrops is responsible for initiation of rapid growth and the forming of massive colonies on host surfaces (8). This has been seen as a method of how these bacterial cells are able to spread infection. This can also however, prevent infection by washing off bacterial cells from yet-infected surfaces.
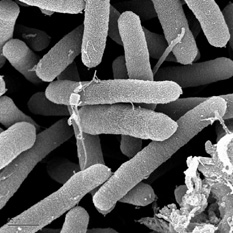
Pseudomonas syringae shown using SEM. Source: Gordon Vrdoljak, Electron Microscopy Laboratory, U.C. Berkeley [1]
Importance
This particular species of Pseudomonas has been the subject of a large array of studies over the past 35 years with its involvement in crop freezing. The majority of frost-sensitive plants usually suffer from damage between -2 and -5oC (11, 12). When water gets this cold, water turns into ice in both inter- and intracellular ways, causing frost damage. P. syringae express a particular type of surface protein, ice-nucleation protein (INP), which increases temperatures to which water is able to freeze. In the absence of sites capable of ice nucleation, the cold water can supercool and freezing will not occur until the temperature is low enough for the most active ice nucleus available is able to catalyze crystallization of supercooled water (freezing)(4). Thus, supercooling instead of ice-nucleation could be a mechanism of frost protection.
P. syringae is a species of bacteria very important to present-day society, particularly with the ever-increasing population of planet earth. Chronic hunger affects 820 million people worldwide, killing 25,000 people every day, one child every eight seconds (13). Large quantities of crops are lost every year are lost due to frost such as in the state of Florida, where 40% of the world’s orange juice supply is grown (14). A large area of interest with Pseudomonas syringae focuses on genetically engineering these bacteria with ice-nucleation-minus proteins. Without these proteins it is thought the temperatures tolerable by plants may be decreased.
Ice Nucleation Active (INA) Proteins
Description
All proteins interact with water, but there are two classes in particular which have a function relating to ice: antifreeze proteins (AFPs) and ice-nucleation proteins (INPs). AFPs have particular structures known to inhibit formation of ice crystals, while INPs do just the opposite. INPs can be seen affiliated with the production of artificial snow (8). Pure water can be supercooled to -40oC in the absence of a heteronucleus (9). INPs are able to promote ice formation by raising the nucleation temperature, and in vitro this temperature can range from -14 to -2oC depending on the number of proteins that cluster together.
Structure
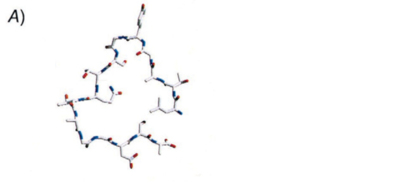
Graether and Jia (2001) attempted to present a model of INP from Pseudomonas syringae based on comparison with two newly determined insect AFP structures. They analyzed the INP sequence of ~60 16-residue repeats similar to a different model organism, and proposed a 16-residue loop for P. syringae (Picture 3). Their result suggested that insect AFPs and bacterial INPs may have a similar B-helical structure, even though they have opposite effects on water molecules.
Bacterial Effects
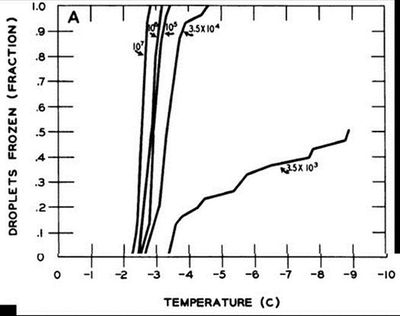
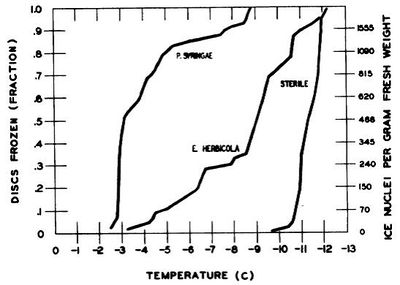
Beginning more than 25 years ago, scientists began noticing that concentrations of bacteria residing on leaf surfaces were correlated with the temperatures at which freezing occurred in plant tissues. A study by Lindow et al., (1982) concluded that higher concentrations of P. syringae on leaf surfaces were associated with warmer temperatures of freezing (Figure 1). As concentrations of ice-nucleating active cells in water droplets increased, higher temperatures of freezing were seen. At concentrations of 3.5 x 103 Pseudomonas syringae cells/ml, less than half water droplets were still unfrozen at -9oC. They also showed that freezing temperatures were much lower without the presence of the bacteria on the surface of plant tissues (Figure 2). When concentrations of P. syringae were sprayed onto corn leaf disks half of the disks were frozen by temperatures of -3oC, whereas when sterilized water was used, half disks were unfrozen until -11oC. These results led researchers to conclude that bacteria on the surfaces of leaves play a major role in plant freezing.
Problems
In mutant lines of P. syringae which are ice-nucleation inactive (Ice-) have been genetically engineered and used in a variety of studies (22). Competition for limiting resources such as space and nutrients on leaf surfaces was suggested as a mechanism by which Ice- bacteria may prevent buildup of incoming Ice+ strains. With the use of potato plants, reduced population sizes of Ice+ bacteria treated with Ice- strains correlated with reduced amounts of frost injury during a natural radiative forest event. Thus, biologists are concerned that an introduction of bacteria with genetic mutation on the INA proteins might be detrimental to the survival of the original species.
Current Research
Clouds
The use of ice-nucleating bacteria has already been seen in uses for creating fake snow to protection of crops from frost during growing seasons. On a larger scale, IN bacteria has begun to be studied in the atmosphere. Mohler, et al. (2008) conducted a study investigating five different bacterial species in the range of -5 to -15oC and their correlations to condensation. Pseudomonas syringae resides on plant surfaces, where it is emitted into the atmosphere (17). Ice nucleation active strains have been detected in rain and snow, as well as in the atmosphere (18, 19), and suggests that they may be disseminated through the water cycle. Bacterial ice nucleation is an important area of study in the initiation of precipitation, and with particular species nucleation can occur at a range of temperatures. In this study, bacterial cells were suspended in the aerosol phase using the Aerosol Interaction and Dynamics in the Atmosphere facility in Germany. Growing water droplets and ice particles were sensitively detected using in situ light scattering and depolarization setup SIMONE, which measures the depolarization of polarized light at a particular wavelength scattered from particles in the center of the large, cold cloud chamber. Infrared extinction spectrometers were also used to characterize droplet and ice clouds. As a result, the IN active cell fraction was calculated from the ratio of ice particle number concentration to number of total cells. The bacteria investigated are mainly IN active between -7 and -11oC with an IN active fraction of the order of 10-4. This means that in similar conditions to what is present in the atmosphere, IN active bacteria are able to form ice crystals. From this data, future studies should test at what concentrations of IN active bacterial cells the initiation of precipitation occurs through the ice phase. More studies are needed to measure distribution, sources, and bacterial cell concentration in the troposphere, as well as characterization of IN active cells extracted from cloud and rain water.
Foods
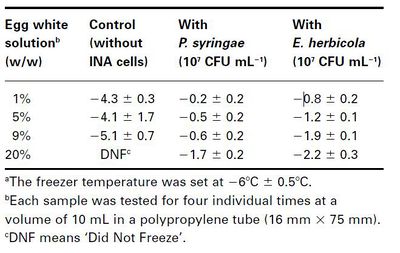
There have also been studies using ice-nucleating bacteria commercially. In some uses, bacterial ice nucleation at higher temperatures may be useful in keeping foods frozen. For example, it was found that the nucleation temperature of salmon muscle with the use of ice-nucleation active (INA) Pseudomonas syringae was raised from -4.9 to -1.5oC (21). This means that the difference between the freezing-point temperature and the nucleation temperature of the sample was reduced by 3.4oC (Table XXXX). Li et al. (1997) tests freezing effects in multiple foods in response to concentrations of bacterial ice nucleators. The addition of P. syringae cells to egg white solutions resulted in a significant increase in their nucleation temperatures at -6oC (Table XXXXXX). In solution containing 9% egg white solution freezing occurred at -5.1oC without INA bacteria, but was raised to -0.6oC in the presence of INA Pseudomonas syringae cells. At 20% egg white solution, the control did not freeze at -6oC, but the INA samples had frozen easily. This means that the behavior of the egg white solution was determined by the presence or absence of INA bacteria. It may be impractical to supplement large quantities of bacteria into everyday foods, but their effects on the freezing model of food systems may provide information for future studies of microbes in the food industry.
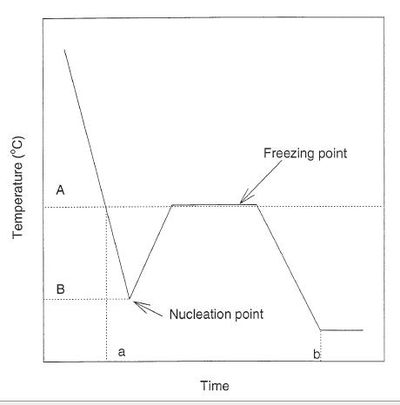
Hypothetical freezing curve of water. Degree of supercooling is defined as the temperature difference between freezing point (A) and nucleation point (B). The total freezing time is the time difference between when the temperature of water passes the freezing point (a), and when it reaches the freezer temperature (b). Source: Li et al., 1997.
Conclusion
Although most go unnoticed, the presence of ice-nucleating bacteria is important for everyday life from agriculture to making snow. In particular, Pseudomonas syringae have been an epiphyte of great interest for nearly three decades. Its ice nucleating proteins protruding from the membrane are important to structural properties of molecule organization. The process of nucleation can be catalyzed by these specific IN proteins, or inhibited by a mutation in these proteins. The addition of ice-nucleating bacteria to agriculture has potential benefits of protecting crops from frosts dropping below freezing, which might contribute to a solution of the world-wide problem of starvation and chronic hunger. These bacterial mechanisms might also someday be used to keep food frozen leading to conservation of energy or other benefits. The role of INA bacteria is also predicted to influence cloud formation. Future studies could uncover even more answers for most people who don’t realize how dependent they really are on these bacteria.
References
[1] http://genome.jgi-psf.org/psesy/psesy.home.html [2]
[2] Steele, H., B.E. Laue, G.A. MacAskill, A.J. Hendry, and S. Green. “Analysis of the natural infection of European horse chestnut (Aesculus hippocastanum) by Pseudomonas syringae pv. Aesculi.” Plant Pathology 59: 1005-1013.
[3] Graether, S.P., and Z. Jia. 2001. Modeling Pseudomonas syringae ice-nucleation protein as a B-helical protein. Biophysical Journal 80: 1169-1173.
[4] Lindow, S.E., D.C. Arny, and C.D. Upper. 1982. Bacterial ice nucleation: a factor in frost injury to plants. Plant Physiology 70: 1084-1089.
[5] Anzai, Y., H. Kim, J. Park, H. Wakabayashi, and H. Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. International Journal of Systematic and Evolutionary Microbiology 50:1563-1589. [6] Kurz, M., A.Y. Burch, B. Seip, S.E. Lindow, and H. Gross. 2010. Genome-driven investigation of compatible solute biosynthesis pathways of Pseudomonas syringae pv. Sytingae and their contribution to water stress tolerance. Applied and Environmental Microbiology 76(16): 5452-5462. [7] http://pseudomonas-syringae.org/ [8] Margaritis, A., and A. S. Bassi. 1991. Principles and biotechnological applications of bacterial ice nucleation. Critical Reviews Biotechnology 11: 277–295. [9] Hobbs, P. V. 1974. Ice Physics. Clarendon Press, Oxford. [10] Dulla, G.F.J., K.V. Krasileva, and S.E. Lindow. 2010. Inference of quorum sensing in Pseudomonas syringae by bacterial epiphytes that limit iron availability. Environmental Microbiology 12(6): 1762-1774. [11] Burke, M.J., L.V. Gusta, H.A. Quamme, C.J. Weiser, and P.H. Li. 1976. Freezing and injury to plants. Annual Reviews of Plant Physiology 27: 507-528. [12] Levitt, J. 1972. Responses of plants to environmental stresses. Academic Press, New York. [13] Stanford, Claire. “World Hunger.” The Reference Shelf, 79(5). The H.W. Wilson Company, 2007. [14] Rottman, karl. “Freeze may take heavy toll on Forida citrus.” CNN US, January 11, 2010. Accessed from http://www.cnn.com/2010/US/weather/01/11/cold.weather/index.html?iref=allsearch. [15] Hirano, S.S., and C.D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae – a pathogen, ice nucleus, and epiphyte. American Society for Microbiology 64(3): 624-653. [16] Lindow, S.E. 1995. Control of epiphytic ice nucleation-active bacteria for management of plant frost injury, p. 239-256. In R.E. Lee, G.J. Warren, and L.V. Gusta, Biological ice nucleation and its applications. American Phytopathological Society, St. Paul, Minnesota. [17] Lindemann, J., H.A. Constantinidou, W.R. Barchet, and C.D. Upper. 1982. Plants as a source of airborne bacteria, including ice nucleation active bacteria. Applications in Environmental Microbiology 44: 1059-1063. [18] Morris, C.E., D.C. Sands, B.A. Vinatzer, C. Glaux, C. Guilbaud, A. Buffiere, S. Yan, H. Dominguez, and B.M. Thompson. 2008. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. The ISME Journal 2: 321-334. [19] Amato, P., M. Parazols, M. Sancelme, P. Laj, G. Mailhot, and A.M. Delort. 2007. Microorganisms isolated from the water phase of trophospheric clouds at the Puy de Dome: major groups and growth abilities at low temperatures. FEMS Microbiological Ecology 59: 242-254. [20] Mohler, O., D.G. Gerrgakopoulos, C.E. Morris, S. Benz, V. Ebert, S> Hunsmann, H. Saathoff, M. Schnaiter. 2008. Heterogeneous ice nucleation activity of bacteria: new laboratory experiments at simulated cloud conditions. Biogeosciences 5: 1425-1435. [21] Ryder, J.M., and T.C. Lee. 1988. Paper presented at the 1988 Annual Meeting of Institute of Food Technologists, New Orleans, Louisiana. Book of Abstracts. Pp151. Institute of Food Technologists, Chicago, Illinois. [22] Wilson, M., and S.E. Lindow. 1994. Ecological similarity and coexistence of epiphytic ice-nucleation (Ice+) Pseudomonas syringae strains and a non-ice-nucleating (Ice-) biological control agent. Applications of Environmental Microbiology 60: 3128-3137.
Edited by Ryan O'Connor,student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.