Nostoc muscorum: Difference between revisions
No edit summary |
|||
| Line 35: | Line 35: | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
<i>Nostoc muscorum</i> is a phototroph, requiring CO2 for growth. It can also utilize glucose and sucrose for growth (Allison et al., 1937). <i>N. muscorum</i> has heterocysts, which are specialized nitrogen-fixation cells. Heterocysts, (5-10% of cells) appear when <i>N. muscorum</i> is transferred to nitrogen free media. Appearance of heterocysts is concurrent with an increase in nitrogenase activity, which reduces N2 to NH3 (Fleming et al.) Magnesium is also required for <i>N. muscorum</i> to carry out nitrogen fixation (Allison et al., 1937). | |||
<i>Nostoc muscorum</i> possesses a motile stage in its life cycle that allows it to maintain populations that follow areas of high light intensity through the use of hormogones, which are motile filaments (Rai et. al, 2002). Formation of hormogonium is sensitive to metabolic products present as well as light intensity (K.Lazaroff, 1963), (Rai et. al, 2002). Hormogonium is formed rapidly when containing glucose and when sucrose is present, the breakdown of organized thalli and formation takes place only after prolonged incubation. (K.Lazaroff, 1963) | |||
<i>Nostoc muscorum</i> are tolerant of saline environments, with sugar products providing osmoregulatory activity in salt tolerance (Blumwald & Tel-Or, 1982). Heterocyst frequency drops immediately following exposure to salty conditions. When these cells collapse, nitrogenase synthesis stops and vegetative cells may go under osmoregulation. New heterocysts eventually recover after a short period of adaptation. Salt adapted vegetative cells about 2x as large as normal cells (Blumwald & Tel-Or, 1982). There are also drastic changes in the intracellular organization of the thylakoidal assembly. Thylakoid membranes, containing photosynthetic pigments, change their distribution patterns in cells when the cell is exposed to salt. Instead of concentrating on the outer edges, they become identically distributed throughout the cell volume (Blumwald & Tel-Or, 1982). | |||
<i>N. muscorum</i>’s life cycle is dependent on the light conditions; cells of a coccoid form grow in the dark (aseriate stage) while cells of a filamentous form grow in light (seriate stage). The conversion from the aseriate to the seriate stage is photocontrolled by red stimulation and green suppression (T.Isono and Y.Fujita, 1981) . <i>N. muscorum</i> has a fully active photosynthetic machinery even when they are grown in the dark. Thus, light can act not only as a signal for the photocontrolled phenomena but also as energy for cell growth. At higher light intensity and cell growth, there are a higher proportion of seriate cells. These features indicate that light mainly acts as the energy source for photosynthesis which enhances the cell growth. (T.Isono and Y.Fujita, 1981) | |||
Revision as of 16:28, 14 April 2012
Classification
Domain: Bacteria
Phylum: Cyanobacteria
Class: Cyanophyceae
Order: Nostocales
Family: Nostocaceae
Genus: Nostoc
Species
|
NCBI: Taxonomy |
Genus species
Description and Significance
Nostoc muscorum is a free-living microorganism which inhabits both terrestrial and freshwater aquatic environments (Cameron, 1960) (Blumwald & Tel-Or, 1982). N. muscorum cells are filamentous, gram-negative green-brown colored algal cells which can form spores under desiccation conditions (Allison et al., 1937). As cyanobacteria, these organisms are phototrophic, performing photosynthesis in their environments and also fixing atmospheric nitrogen (Blumwald & Tel-Or, 1982).
The ideal environment for Nostoc muscorum is one with pH in the range of 7.0 to 8.5. These organisms cannot tolerate a pH lower than 5.7 (Allison et al., 1937). N. muscorum grows best when light intensity is less than that of direct sunlight, but can continue to grow and fix nitrogen for months in the presence of glucose and absence of sunlight. (Allison et al., 1937)
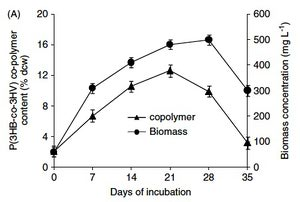
Nostoc muscorum are important for the nutrient cycling of carbon and nitrogen within the soil ecosystems in which they are found. Inoculation with this species has been shown to increase soil C content by 50-63% and N content by 111-120% as compared with uninoculated soil. (Rogers and Burns, 1993) The process of fixing atmospheric nitrogen contributes plant-available nitrogen to the soil, improving plant growth (Rogers and Burns, 1993).
Soils inoculated with N. muscorum also show an increase in soil aggregate resistance to degradation during wetting and physical disruption. (Rogers and Burns, 1993) This soil stability contribution can help prevent soil erosion, contributing to a higher success of seedling emergence in soils containing N. muscorum compared to those without it (Rogers and Burns, 1993) (Cameron, 1960). The aggregate-forming contributions of the microorganism also helps prevent desertification or the erosion of desert soils, where it is often present as part of cyanobacterial algal crusts (Cameron, 1960).
Current research with Nostoc muscorum involves manipulating the cells’ formation of poly (3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] co-polymer as potential materials to use in bioplastics for general and medical applications (Bhati and Mallick, 2012). Nostoc muscorum has the potential to be used in industrial settings which produce CO2 waste by using this waste as fuel under propionate- or valerate-supplemented conditions to form the above polymers for thermoplastic formation (Bhati and Mallick, 2012).
Genome Structure
Describe the size and content of the genome. How many chromosomes? Circular or linear? Other interesting features? What is known about its sequence?
Cell Structure, Metabolism and Life Cycle
Nostoc muscorum is a phototroph, requiring CO2 for growth. It can also utilize glucose and sucrose for growth (Allison et al., 1937). N. muscorum has heterocysts, which are specialized nitrogen-fixation cells. Heterocysts, (5-10% of cells) appear when N. muscorum is transferred to nitrogen free media. Appearance of heterocysts is concurrent with an increase in nitrogenase activity, which reduces N2 to NH3 (Fleming et al.) Magnesium is also required for N. muscorum to carry out nitrogen fixation (Allison et al., 1937).
Nostoc muscorum possesses a motile stage in its life cycle that allows it to maintain populations that follow areas of high light intensity through the use of hormogones, which are motile filaments (Rai et. al, 2002). Formation of hormogonium is sensitive to metabolic products present as well as light intensity (K.Lazaroff, 1963), (Rai et. al, 2002). Hormogonium is formed rapidly when containing glucose and when sucrose is present, the breakdown of organized thalli and formation takes place only after prolonged incubation. (K.Lazaroff, 1963)
Nostoc muscorum are tolerant of saline environments, with sugar products providing osmoregulatory activity in salt tolerance (Blumwald & Tel-Or, 1982). Heterocyst frequency drops immediately following exposure to salty conditions. When these cells collapse, nitrogenase synthesis stops and vegetative cells may go under osmoregulation. New heterocysts eventually recover after a short period of adaptation. Salt adapted vegetative cells about 2x as large as normal cells (Blumwald & Tel-Or, 1982). There are also drastic changes in the intracellular organization of the thylakoidal assembly. Thylakoid membranes, containing photosynthetic pigments, change their distribution patterns in cells when the cell is exposed to salt. Instead of concentrating on the outer edges, they become identically distributed throughout the cell volume (Blumwald & Tel-Or, 1982).
N. muscorum’s life cycle is dependent on the light conditions; cells of a coccoid form grow in the dark (aseriate stage) while cells of a filamentous form grow in light (seriate stage). The conversion from the aseriate to the seriate stage is photocontrolled by red stimulation and green suppression (T.Isono and Y.Fujita, 1981) . N. muscorum has a fully active photosynthetic machinery even when they are grown in the dark. Thus, light can act not only as a signal for the photocontrolled phenomena but also as energy for cell growth. At higher light intensity and cell growth, there are a higher proportion of seriate cells. These features indicate that light mainly acts as the energy source for photosynthesis which enhances the cell growth. (T.Isono and Y.Fujita, 1981)
Ecology and Pathogenesis
Habitat; symbiosis; biogeochemical significance; contributions to environment.
If relevant, how does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
References
[Blumwald, E., E. Tel-Or. “Structural aspects of the adaptation of Nostoc muscorum to salt.” Archives of Microbiology. 1982. volume 132. p.163-167.]
Cameron, R.E. "Communities of Soil Algae Occurring in the Sonoran Desert in Arizona". Journal of the Arizona Academy of Science. 1960. Volume 1, No. 3. p. 85-88. [1]
[Fleming, H., R. Haselkorn. "Differentiation in Nostoc muscorum: Nitrogenase Is Synthesized in Heterocysts (nitrogenase induction/cellular differentiation/polyacrylamide-gel electrophoresis)". PNAS 1973. Volume 70, No. 10, p. 2727-2731]
Isono.T and Fujita.Y "Studies on Morphological Changes of the Blue-green Alga Nostoc muscorum A with Special Reference to the Role of Light". <ii>Plant & Cell Physiology. 1981. Volume 22, No. 2. p. 185-195.
[Rai, N.A., B. Bergman, U. Rasmussen. “Cyanobacteria in Symbiosis.” Springer Publishers 2002.]
Author
Page authored by _____, student of Prof. Jay Lennon at Michigan State University.
<-- Do not remove this line-->
