Foaming in wastewater treatment plant (WWTP): Difference between revisions
| Line 45: | Line 45: | ||
===Substrate Storage=== | ===Substrate Storage=== | ||
<i>M. parvicella</i> and <i>Mycolata </i>were reported to be able to utilize various organic compounds as carbon and energy source. The compounds contain organic acids, complex substrates and fatty acids under aerobic, anoxic and anaerobic conditions. The substrates can then be storaged intracellularlly in filamentous bacterium. Intracellular storage of poly β-hydroxyalkanoates(PHA)like inclusions were observed in aerobically-grown <i> M. parvicella </i> under anoxic or anaerobic conditions[3]. Lipid storage granules were also observed in some <i> M. parvicella </i> from activated sludge in nutrient removal WWTP [5]. <i>Mycolata </i> were also found capable in forming intracellular PHA storage [6]. The storage capability of filamentous bacteria allows them survive in harsh conditions during operation(e.g. substrates-limiting in foam, alternating anaerobic-aerobic environment), and out-compete floc-forming and other bacteria in activated sludge, most of which can not uptake and storage substrates anaerobically. | <i>M. parvicella</i> and <i>Mycolata </i>were reported to be able to utilize various organic compounds as carbon and energy source. The compounds contain organic acids, complex substrates and fatty acids under aerobic, anoxic and anaerobic conditions. The substrates can then be storaged intracellularlly in filamentous bacterium. Intracellular storage of poly β-hydroxyalkanoates(PHA) like inclusions were observed in aerobically-grown <i> M. parvicella </i> under anoxic or anaerobic conditions[3]. Lipid storage granules were also observed in some <i> M. parvicella </i> from activated sludge in nutrient removal WWTP [5]. <i>Mycolata </i> were also found capable in forming intracellular PHA storage [6]. The storage capability of filamentous bacteria allows them survive in harsh conditions during operation(e.g. substrates-limiting in foam, alternating anaerobic-aerobic environment), and out-compete floc-forming and other bacteria in activated sludge, most of which can not uptake and storage substrates anaerobically. | ||
===Cell Surface Hydrophobicity and Exoenzyme Activities=== | ===Cell Surface Hydrophobicity and Exoenzyme Activities=== | ||
Revision as of 18:56, 20 April 2012
Introduction
Foaming in activated sludge process is a common operational problem in many wastewater treatment plants. The foam can occur in aeration tank, secondary clarifier, as well as in anaerobic digester. Foam in WWTP is normally sticky, viscous and brown in color. It floats and accumulates on top of the tanks, and can take up a large fraction of solids inventory and reactor volume, thus decreasing the effluent quality and control of sludge retention time (SRT). The foam can also overflow onto walkways and surrounding areas, posting severe difficulties and risk to operation and environment.

Many reasons are associated with foaming: presence of slowly biodegradable surfactants (eg. household detergents) from industrial or municipal wastewater, excess production of extracellular polymeric substance (EPS) by activated sludge microorganisms under nutrient-limited condition, proliferation of filamentous organisms and gas provided in aeration tank or produced in anoxic zone of aeration tanks, secondary clarifiers and anaerobic digesters.
Stable foaming in WWTP is the product from interaction among gas bubble, surfactant and hydrophobic particles. The hydrophobic particles congregate at the air-water interface and strengthen the water film between air bubbles. Meanwhile, the particles also serve as collector for surfactant which stabilizes the foam. Gas bubbles in WWTP are generated by aeration, mechanic mixing and biological process like denitrification and anaerobic digestion; Surfactants in WWTP come from the wastewater streams that contain slowly biodegradable surfactants; hydrophobic particles are normally referred to the filamentous bacteria with a long-chain structure and hydrophobic surface [1].
Physical and chemical environment
Gas bubbles
From the mechanism for foaming mentioned above, we know gas bubbles are essential in foam generation. Gas bubbles are involved in many steps of activated sludge process. In aeration tank, aeration and mechanic mixing is employed to ensure enough dissolved oxygen for aerobic degradation of organic pollutants or nitrification. This thus creates abundance of gas bubbles. Other than external introduction by aeration or mixing, gas bubbles can also be produced from biological processes themselves. Both denitrification in secondary clarifier and anaerobic digestion in digester produce gas like N2 or CH4, CO2. These gases favor the generation of foam.
Surfactants
Most surfactants in WWTP originate from the detergents, oil and grease that used in households or industry. The EPS produced by bacteria is also believed to contribute part of the surfactants. Surfactant could stabilize the foaming and allow foam to accumulate. Ho and Jenkins demonstrated the conducive effect of a slowly biodegradable nonionic surfactant in foaming [1,2].
pH and Temperature
Filamentous bacteria are important in forming stable foam. The growth rate of filamentous bacteria was found not affected significantly for range of pH from 6.7 to 8.0, only slightly decreased at pH 8.4. Optimum temperature of Microthrix parvicella , a filamentous bacterium associated with foam production, was reported around 25 oC, some growth was observed at 8 oC, and poor or no growth at above 35 oC [3].
Dissolved oxygen (DO)
Filamentous bacterium M. parvicella prefer low oxygen concentration, and were found proliferate in WWTP with spatial or temporal low DO. In Ekama’s study,M. parvicella was eliminated by increasing DO to 2-3 mg/L[4]. As an effective control for sludge bulking and foaming, aerobic selectors with high DO (>2mg/L) are often placed before aeration tank to inhibit the growth of filamentous bacteria[3].
Mean Cell Retention Time (MCRT)
More filamentous bacteria were observed in WWTP and studies when increasing MCRT (1.5 to 20 d), while MCRT at around 1 d was effective in limit growth of filamentous bacteria. Controlling MCRT sometimes could be difficult to achieve by increasing flow rate of water since biomass could be retained in foam without moving with water[1,3].
Key Microorganisms
Filamentous bacteria serve as hydrophilic particles which play important role in stabilizing foaming in WWTP. There are two main groups of filamentous bacteria: the most commonly observed group: Candidatus Microthix parvicella , and members of the Mycolata.
Microthix parvicella
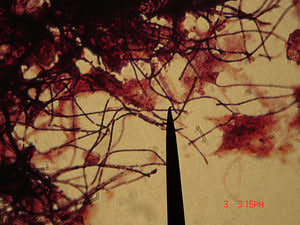
M. parvicella are gram-positive nonbranched filamentous bacteria. They are aerobic, non-fermentative and can reduce nitrate. Although M. parvicella can grow over a wide range of oxygen concentration, they prefer microaerophilic conditions for good growth. The filaments they produced at low DO (~ 0.4 mg/L) are long and regular without empty or deformed cells that observed under high DO conditions[3,5].
Mycolata
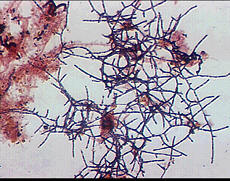
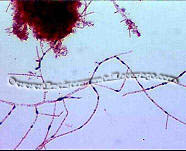
Mycolata, often referred to as "nocardia", are a group of filamentous bacteria that contain mycolic acids in their cell walls. They are under the order Actinomycetales in phylum Actinobacteria, isolates were identified as member in families Corynebacteriaceae, Dieziaceae, Gordoniaceae, Mycobacteriaceae, Nocardiaceae, Tsukamurellaceae and Williamsiaceae. They have two major morphotypes: one with right-angled branching pattern and the other acute-angled branching pattern. Mycolata were found to uptake a wide range of organic compounds, and can use nitrate or nitrite as electron acceptor. Many mycolata can store polyhydroxyalkanoate in cell and present high cell surface hydrophobicity[6].
Microbial Processes
Substrate Storage
M. parvicella and Mycolata were reported to be able to utilize various organic compounds as carbon and energy source. The compounds contain organic acids, complex substrates and fatty acids under aerobic, anoxic and anaerobic conditions. The substrates can then be storaged intracellularlly in filamentous bacterium. Intracellular storage of poly β-hydroxyalkanoates(PHA) like inclusions were observed in aerobically-grown M. parvicella under anoxic or anaerobic conditions[3]. Lipid storage granules were also observed in some M. parvicella from activated sludge in nutrient removal WWTP [5]. Mycolata were also found capable in forming intracellular PHA storage [6]. The storage capability of filamentous bacteria allows them survive in harsh conditions during operation(e.g. substrates-limiting in foam, alternating anaerobic-aerobic environment), and out-compete floc-forming and other bacteria in activated sludge, most of which can not uptake and storage substrates anaerobically.
Cell Surface Hydrophobicity and Exoenzyme Activities
Higher cell surface hydrophobicity were found in cells of M. parvicella and Mycolata than other bacteria in activated sludge. The more hydrophobic cell surface enable filamentous bacteria better attraction to hydrophobic substrates like lipids, long-chain fatty acid (LCFA). Additionally, filametnous bacteria produce many exoenzymes such as lipases, which enhance the degradation and utilization of substrates [3,6].
Foaming Control Strategy
There are no universal strategies effective for foaming control in WWTP. Specific measures need to be taken according to the reason of foaming, organisms involved and operational condition. Common strategies for foaming control include: Reduction of SRT (Sludge Retention Time, similar to mean cell retention time, often used in wastewater treatment operation) to wash out filamentous bacteria; removal of hydrophobic substances and substrate that could enhance foaming or favor the growth of filamentous bacteria; introduction of selectors before aeration tanks to suppress the growth of filamentous bacteria; addition of oxidizing agents like chlorine to kill filamentous bacteria (chlorine also kills other bacteria)[1,2,3].
Current Research
Identification of Filamentous Bacteria
Traditional identification of filamentous bacteria relies on their morphology under microscopy. However, many filamentous bacteria may not have distinguishable morphology, therefore, identification based on 16S or 23S rRNA genes are preferred. Nielsen group from Denmark developed more effective permeabilization protocol for fluorescence in-situ hybridization (FISH) that could enhance the hybridization and produce stronger signal. They performed a various ecophysiology studies on different filamentous bacteria from foam and activated sludge sample using MAR-FISH[6]. Other 16S-based techniques like PCR-DGGE were also employed in detection of filamentous bacteria[7].
Development of Effective Foaming-Control Chemicals
Conventional oxidizing chemicals like chlorine that used to destroy filamentous bacteria also affect the growth of other bacteria in activated sludge. More selective chemicals for controlling filamentous bacteria are desired. Polyaluminium chloride (PAX-14) was found effective in controlling the foaming caused by M. parvicella. Addition of PAX-14 did not affect the nitrification and COD removal performance. However, the mechanism PAX-14 in controlling M. parvicella is still not clear[8].
Mechanism of Foaming
Petrovski et. al closely examined the role of surfactant in foaming based on the data from 65 foaming Mycolata. They found the floatation theory can be applied in explaining the role of surfactant in activated sludge foaming. Mycolata without surfactant could produce scum, while presence of surfactant without hydrophobic particle created unstable foam. They also found Bacillus subtilis, commonly culturable from foam could play important role in foaming with its production of surface surfactant[9].
References
[1]Jenkins, D, Richard, MG, Daigger, GT. 2004. “Manual on the Causes and Control of Activated Sludge Bulking, Foaming, and Other Solids Separation Problems”, 3rd edition.. Lewis Publishers, New York. http://www.iwapublishing.com/template.cfm?name=isbn1843390469
[2]Hug T. 2006. “ Characterization and controlling of foam and scum in activated sludge systems”. ETH Ph.D. Dissertation. http://dx.doi.org/10.3929/ethz-a-005180592
[3] Rossetti S, Tomei MC, Nielsen PH, Tandoi T. 2005. “ ‘Microthrix parvicella’, a filamentous bacterium causing bulking and foaming in activated sludge systems: a review of current knowledge”. FEMS Microbiology Reviews, 29(1): 49–64. http://www.sciencedirect.com/science/article/pii/S016864450400066X
[4]Ekama, GA Marais, GVR. 1984. “Theory, Design and Operation of Nutrient Removal Activated Sludge Processes”, Water Research Commission. 5.1–5.18, Pretoria.
[5] Nielsen, PH, Roslev, P, Dueholm, T, Nielsen, JL. 2002. “Microthrix parvicella, a specialized lipid consumer in anaerobic –aerobic activated sludge plants”. Water Sci. Technol. 46: 73–80.
[6] Kragelund C, Remesova Z, Nielsen JL, Thomsen TR, Eales K, Seviour R, Wanner J, Nielsen PH. 2007. “Ecophysiology of mycolic acid-containing Actinobacteria (Mycolata) in activated sludge foams”. FEMS Microbiol Ecol. 61(1):174-84. http://www.ncbi.nlm.nih.gov/pubmed/17466023
[7] Shen FT, Huang HR, Arun AB, Lu HL, Lin TC, Rekha PD, Young CC. 2007. “Detection of filamentous genus Gordonia in foam samples using genus-specific primers combined with PCR--denaturing gradient gel electrophoresis analysis”. Can J Microbiol. 53(6):768-74. http://www.ncbi.nlm.nih.gov/pubmed/17668037
[8]Roels, T, Dauwe, F, Van Damme, S, De Wilde, K,Roelandt, F. 2002. “ The influence of PAX-14 on activated sludge systems and in particular on Microthrix parvicella”. Water Sci.Technol. 46(1-2), 487–490. http://www.ncbi.nlm.nih.gov/pubmed/12216672
[9]Petrovski S, Dyson ZA, Quill ES, McIlroy SJ, Tillett D, Seviour RJ. 2011. “An examination of the mechanisms for stable foam formation in activatedsludge systems”. Water Research. 45 (5): 2146–2154. http://www.sciencedirect.com/science/article/pii/S004313541000881X
Edited by Weiwei Wu, a student of Angela Kent at the University of Illinois at Urbana-Champaign.
