Ebola Transmission: Difference between revisions
Vgawlik6782 (talk | contribs) No edit summary |
Vgawlik6782 (talk | contribs) No edit summary |
||
| Line 3: | Line 3: | ||
The Ebola virus is a relatively recently described, severe disease-causing pathogen that poses a huge threat to human health mostly within central Africa. This is, in part, due to its high mortality<sup>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1579243/ 4]</sup> and lack of affordable treatment options. Ebola is considered a Biosafety level 4 (BSL-4) agent; classifying it among the most threatening pathogens that exist in the world today. Agents within this category pose severe threats to human health and can be fatal due to the lack of available vaccines. There are five known Ebola species within the family <i>Filoviridae</i> and all of the species within the family cause varying degrees of viral hemorrhagic fever illnesses.<sup>[4]</sup> | The Ebola virus is a relatively recently described, severe disease-causing pathogen that poses a huge threat to human health mostly within central Africa. This is, in part, due to its high mortality<sup>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1579243/ 4]</sup> and lack of affordable treatment options. Ebola is considered a Biosafety level 4 (BSL-4) agent; classifying it among the most threatening pathogens that exist in the world today. Agents within this category pose severe threats to human health and can be fatal due to the lack of available vaccines. There are five known Ebola species within the family <i>Filoviridae</i>, four of them endemic to Africa<sup>[6]</sup>, and all of the species within the family cause varying degrees of viral hemorrhagic fever illnesses.<sup>[4]</sup> | ||
| Line 10: | Line 10: | ||
<br> | <br> | ||
[[Image:Ebolavirus.jpg|thumb|400px|left|A closeup photo of the Ebola virus.]]The Ebola virus has an RNA genome that codes for at least seven proteins and is enclosed in a nucleocapsid<sup>[3]</sup>. Four of the virus’ proteins are thought to comprise the capsid. The capsid allows the transcription and the translation of the viral genome and is therefore the principle player in the virus’ pathogenicity<sup>[4]</sup>. In addition, the virus has been shown to impair the immune function of the body by way of its infection of the phagocytic cells. The nucleocapsid and viral proteins move to new sites of infection and form themselves into virons where they can then go on to infect around the host body.<br><br> | [[Image:Ebolavirus.jpg|thumb|400px|left|A closeup photo of the Ebola virus.]]The Ebola virus has an RNA genome that codes for at least seven proteins and is enclosed in a nucleocapsid<sup>[3]</sup>. Four of the virus’ proteins are thought to comprise the capsid. The capsid allows the transcription and the translation of the viral genome and is therefore the principle player in the virus’ pathogenicity<sup>[4]</sup>. In addition, the virus has been shown to impair the immune function of the body by way of its infection of the phagocytic cells. The nucleocapsid and viral proteins move to new sites of infection and form themselves into virons where they can then go on to infect around the host body.<br><br> | ||
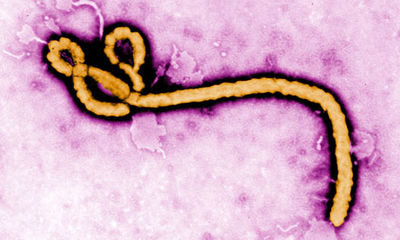
The virus forms many long rods and is much longer than it is wide and is often photographed in a hooked or curved form. The virus is around 80nm in diameter and can be of varying lengths ranging from 600-1400nm<sup>[1]</sup>. Ebola does not produce its own cell membrane and instead steals some of its host membrane to survive. <sup>[4]</sup><sup>[2]</sup><br><br> | The virus forms many long rods and is much longer than it is wide and is often photographed in a hooked or curved form. The virus is around 80nm in diameter and can be of varying lengths ranging from 600-1400nm<sup>[1]</sup>. Ebola does not produce its own cell membrane and instead steals some of its host membrane to survive. <sup>[4]</sup><sup>[2]</sup><br><br> | ||
In recent study it has been indicated that the Ebola virus has developed several customized ways to enter host cells depending on the virus' size and what kind of cell the virus wants to gain access to<sup>[1]</sup>. However, it seems in most cases the virus prefers to use macropincytosis, a form of endocytosis, which allows the cell to steal some of the hosts' membrane as it leaves. It seems that mononuclear phagocytes, hepatocytes, and endothelial cells are the cells that are preferred targets of the infection. | In recent study it has been indicated that the Ebola virus has developed several customized ways to enter host cells depending on the virus' size and what kind of cell the virus wants to gain access to<sup>[1]</sup>. However, it seems in most cases the virus prefers to use macropincytosis, a form of endocytosis, which allows the cell to steal some of the hosts' membrane as it leaves. It seems that mononuclear phagocytes, hepatocytes, and endothelial cells are the cells that are preferred targets of the infection. | ||
<br> | |||
==Containment and Transmission== | ==Containment and Transmission== | ||
[[Image:CDC scientists.JPG|thumb|400px|left|Workers from the Center for Disease Control (CDC) demonstrating proper attire worn in BSL-4 labs.]] | [[Image:CDC scientists.JPG|thumb|400px|left|Workers from the Center for Disease Control (CDC) demonstrating proper attire worn in BSL-4 labs.]] | ||
[[Image:Ebola Outbreaks.jpg|thumb|400px|right|A map indicating the locations of Ebola outbreaks throughout Africa.]] | [[Image:Ebola Outbreaks.jpg|thumb|400px|right|A map indicating the locations of Ebola outbreaks throughout Africa.]] | ||
<i>Containment</i> | |||
<br>Due to its classification as a level four safety agent, studying<br> | <br>Due to its classification as a level four safety agent, studying<br> | ||
Ebola in the lab is no small feat. Scientists who choose to work with <br> | Ebola in the lab is no small feat. The virus is not only well adapted <br> | ||
to infect its hosts, but also excels at spreading from host to host. <br> | |||
Scientists who choose to work with <br> | |||
Ebola must use the utmost caution to prevent the virus' ability to infect<br> | Ebola must use the utmost caution to prevent the virus' ability to infect<br> | ||
them while they conduct their experiments. <br> | them while they conduct their experiments. Full hazard suits must be<br> | ||
worn at all times and each suit is throughly examined before entering<br> | |||
a containment space.<br> | |||
<br> | |||
<i>Transmission</i> | |||
In the past it was thought that the virus only infected and caused death <br> | |||
in humans and non-human primates mostly in the central African region. <br> | |||
The main reservoirs of infection was<br> | |||
thought to be the fruit bat<sup>[6]</sup>. But, in recent years, as more <br> | |||
study is being conducted on the virus it seems that there is more to its <br> | |||
complex interactions among multiple species. It was found that Ebola can,<br> | |||
and does, infect pigs and can pass on to nonhuman primates.<sup>[6]</sup><br> | |||
This is an early sign that pigs may play a role in human infection processes.<br><br> | |||
Because infection can occur due to interaction with tainted bodily fluids<br> | |||
communities most at risk are those without sewage systems and are often<br> | |||
poor areas in developing countries. | |||
<br><br><br><br><br><br><br> | <br><br><br><br><br><br><br> | ||
Revision as of 13:42, 26 March 2013
Introduction
The Ebola virus is a relatively recently described, severe disease-causing pathogen that poses a huge threat to human health mostly within central Africa. This is, in part, due to its high mortality4 and lack of affordable treatment options. Ebola is considered a Biosafety level 4 (BSL-4) agent; classifying it among the most threatening pathogens that exist in the world today. Agents within this category pose severe threats to human health and can be fatal due to the lack of available vaccines. There are five known Ebola species within the family Filoviridae, four of them endemic to Africa[6], and all of the species within the family cause varying degrees of viral hemorrhagic fever illnesses.[4]
Virus Structure and Infection
The Ebola virus has an RNA genome that codes for at least seven proteins and is enclosed in a nucleocapsid[3]. Four of the virus’ proteins are thought to comprise the capsid. The capsid allows the transcription and the translation of the viral genome and is therefore the principle player in the virus’ pathogenicity[4]. In addition, the virus has been shown to impair the immune function of the body by way of its infection of the phagocytic cells. The nucleocapsid and viral proteins move to new sites of infection and form themselves into virons where they can then go on to infect around the host body.
The virus forms many long rods and is much longer than it is wide and is often photographed in a hooked or curved form. The virus is around 80nm in diameter and can be of varying lengths ranging from 600-1400nm[1]. Ebola does not produce its own cell membrane and instead steals some of its host membrane to survive. [4][2]
In recent study it has been indicated that the Ebola virus has developed several customized ways to enter host cells depending on the virus' size and what kind of cell the virus wants to gain access to[1]. However, it seems in most cases the virus prefers to use macropincytosis, a form of endocytosis, which allows the cell to steal some of the hosts' membrane as it leaves. It seems that mononuclear phagocytes, hepatocytes, and endothelial cells are the cells that are preferred targets of the infection.
Containment and Transmission
Containment
Due to its classification as a level four safety agent, studying
Ebola in the lab is no small feat. The virus is not only well adapted
to infect its hosts, but also excels at spreading from host to host.
Scientists who choose to work with
Ebola must use the utmost caution to prevent the virus' ability to infect
them while they conduct their experiments. Full hazard suits must be
worn at all times and each suit is throughly examined before entering
a containment space.
Transmission
In the past it was thought that the virus only infected and caused death
in humans and non-human primates mostly in the central African region.
The main reservoirs of infection was
thought to be the fruit bat[6]. But, in recent years, as more
study is being conducted on the virus it seems that there is more to its
complex interactions among multiple species. It was found that Ebola can,
and does, infect pigs and can pass on to nonhuman primates.[6]
This is an early sign that pigs may play a role in human infection processes.
Because infection can occur due to interaction with tainted bodily fluids
communities most at risk are those without sewage systems and are often
poor areas in developing countries.
Possible Treatments
Include some current research in each topic, with at least one figure
Conclusion
In today's world Ebola is still a huge problem that faces many developing nations within Africa. In 2012 alone there were 5 outbreaks of Hemorrhagic Fevers around the world and 3 of them were due to the Ebola virus.7
References
3. "Ebola virus: from discovery to vaccine". Nature Reviews Immunology 3. 2003. p. 667- 85.
Edited by (Victoria Rose Gawlik), a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.