Fungal Endophytes: Drought Tolerance in Plants
It has been estimated that over 80% of terrestrial plants form a symbiotic association with fungi.[1] Fungal endophytes have played an essential role in the evolution of land plants and remain an important component of terrestrial ecosystems. In these mutualistic associations, fungi may benefit their host plant by acquiring nutrients, increasing plant biomass, and conferring tolerance to biotic and abiotic stresses. Studies show that symbiotic fungi can enhance drought, salt, and soil temperature tolerance of their host plant in addition to increasing its resistance to parasitic fungi and herbivores. These habitat-adapted symbioses enable plants to thrive in harsh conditions where they would otherwise not be able to grow.
It is hypothesized that phototroph-fungi associations enabled plants to first colonize land. The mutualistic association of algae and fungi could have helped them avoid desiccation, damaging solar radiation, and more extreme temperatures.[2] Fungal endophytes today colonize a variety of both monocot and eudicot plants which suggests this symbiosis predates the monocot-dicot split that occured 140-150 Myr ago.[3]
Fungal endophytes have many potential applications in agriculture and conservation, yet there is still much that is not known about plant-fungi symbiosis and the mechanisms behind it. Fungal endophytes alter plants’ growth, development, and root morphology to reduce water consumption and increase nutrient uptake. This may be used to increase crop yield in arid climates and mitigate the negative effects of climate change.
Classes of Fungal Endophytes
Clavicipitaceous Endophytes (C-endophytes)
Class 1
C-endophytes infect the plant shoots of some grasses, form systemic intercellular infections, and are passed on through vertical and horizontal transmission.[4] Many produce alkaloids to protect their host plant from herbivory by insects and mammals, and studies have shown them to confer drought and metal tolerance.[4][5] Endophytes may increase the development of root systems or the length of root hairs.[4]
Nonclavicipitaceous Endophytes (NC-endophytes)
Class 2
Class 2 endophytes are usually found in the roots, stem, or leaves of their hosts.[4] They can be transmitted either vertically through the seed coat or horizontally. [4] They can confer habitat-specific stress tolerance to their hosts, and they infect a higher percentage of plants in high-stress environments.[4]
Class 3 and 4
Class 3 colonizes the shoot of plants while Class 4 colonizes plant roots.[4] Few studies have been performed on Classes 3 and 4 endophytes, and little is known about their ecological role and their ability to confer tolerance.
Reaction to Stress
Osmotic protection
Redman et al (2015) examined the osmotic concentrations in non symbiotic and heat-stress tolerant symbiotic plants. The pattern was different between the two groups, leading them to conclude that symbiotic plants do not only rely on increasing their osmolyte concentrations.[6] Endophyte known to promote drought tolerance have high levels of loline alkaloids. [7] Future experiments could test if these are present in sufficient concentration to prevent the denaturation of macromolecules or reduce the number of reactive oxygen species. [7
Reactive Oxygen Species
An early response to stress is the generation of reactive oxygen species. [6]
Testing Endophyte Conferred Tolerances
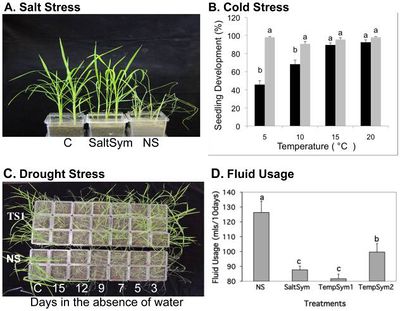
]
Redman et al (2015) tested how well the class 2 fungal endophytes Fusarium culmorum (SaltSym) and Curvularia protuberant (TempSym) would confer rice plants tolerance to salt, drought and cold. SaltSym was isolated from the coastal plant Leymus mollis which is exposed to high salt stress, while TempSym 1 and 2 were isolated from Dichanthelium lanuginosum which grows in geothermal soils.
The study found that plants infected with the endophytes (S) showed no cost when grown in non-stressful conditions, but the number of colonized plants decreased from 100% to 65%.[6]]] When infected plants were grown in stressful environments, their water consumption decreased by 20–30% while their growth rate, reproductive yield, and biomass increased (Figure 1). [6]]] Non-infected plants (NS), on the other hand, lost shoot and root biomass when exposed to stress. All three endophytes treatments took 2-3 times longer to wilt than the non-infected; however, the mechanism of the conferred drought tolerance remains unknown. An interesting observation was that the endophytes changed the development of the plants to increase root biomass before shoot growth.
All plant species used in this experiment are members of the family Poacea but belong to different subfamilies. [6] The isolated fungal endophytes successfully conferred drought tolerance to the rice plants which supports the idea that the symbiotic communication needed to communicate between the fungi and the plant was conserved within the family. [6] While many fungal endophytes show habitat-adapted symbiosis, the fact that there is still lower biodiversity in high stress environments indicates that having the endophyte itself is not enough. [6]
Further Reading
References
|[1][Smith, S., Read, D., 1997: Mycorrhizal symbiosis, 2nd edn., Academy Press, San Diego. ]
|[2]Selosse, M-A, and F. Le Tacon. "The Land Flora: A Phototroph-Fungus Partnership?" Trends in Ecology & Evolution 13.1 (1998): 15-20. Print.
|[3] Shu-Miaw C., Chien-Chang C., Hsin-Liang C., Wen-Hsiung L."Dating the Monocot–Dicot Divergence and the Origin of Core Eudicots Using Whole Chloroplast Genomes". "Journal of Molecular Evolution". 2004. Volume 58, p. 424-441
|[4]Rodriguez, R. J., et al. "Fungal Endophytes: Diversity and Functional Roles." New Phytologist 182.2 (2009): 314-30. Print.
|[5] Koulman, Albert, et al. "Peramine and Other Fungal Alkaloids are Exuded in the Guttation Fluid of Endophyte-Infected Grasses." Phytochemistry 68.3 (2007): 355-60. Print.
|[6] Redman, Regina S. et al. “Increased Fitness of Rice Plants to Abiotic Stress Via Habitat Adapted Symbiosis: A Strategy for Mitigating Impacts of Climate Change.” Ed. Hany A. El-Shemy. PLoS ONE 6.7 (2011): e14823. PMC. Web. 24 Mar. 2015.
|[7] Schardl, C. L., Leuchtmann, A. & Spiering, M. J. (2004) Annu. Rev. Plant Biol. 55, 315-340.
Edited by (Sarah Barnes), a student of Nora Sullivan in BIOL168L (Microbiology) in The Keck Science Department of the Claremont Colleges Spring 2014.
