How E. aerogenes Affects Circadian Rhythm
Circadian Rhythm
By Olivia Smith
Circadian rhythm is a natural endogenous process that regulates the sleep-wake cycle in an organism [1]. This clock cycle is driven by an internal diurnal oscillator set for a period of 24 hours [2]. Circadian rhythms regulate behavior, organs, specifically the gut, and cells in living organisms [3]. External environmental cues, including light, temperature, and redox cycles, can adjust the cycle [1]. Organisms evolved to develop regulatory clock cycles to adapt to the circadian nature of their environment [2]. To competitively survive, as well as optimize function and health, organisms have accommodated rhythmic environmental challenges [1]
. Animals, bacteria, fungi, and plants all exhibit diurnal oscillations of varying degrees of complexity [2]
. This page explains the bidirectional relationship between daily fluctuations of gut microbiota and its host.
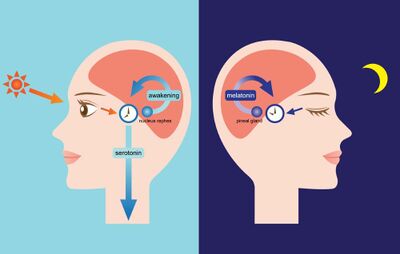
The circadian rhythm is linked to the light/dark cycle [4]. The suprachiasmatic nucleus drives the light/dark cycle in the hypothalamus [2]. In mammals, the primary circadian clock is located in the suprachiasmatic nucleus in the hypothalamus [2]. The suprachiasmatic nucleus generates the clock cycle and synchronizes the periphery [2]. The cycle is synchronized to solar time by retinal afferents from intrinsically photoreceptive retinal ganglion cells [2]. The eye’s retina contains photoreceptive ganglion cells that are photoreceptive and project to the suprachiasmatic nucleus [2].
Ganglion cells contain melanopsin, a photopigment, and signals down a pathway to the suprachiasmatic nucleus [5]. The information from the retinal ganglion cells interprets the lengths of the day and night and passes it to the pineal gland in the epithalamus [5]
. The pineal secretes the hormone melatonin. Melatonin secretion peaks at night and ebbs during the day [9]. Pineal melatonin feeds back to suprachiasmatic nucleus rhythmicity to modulate circadian cycles [9].
There are proteins in the suprachiasmatic nucleus that activate the transcription of genes encoding the repressors PERIOD and CRYPTOCHROME [1]. The products of these genes can form a repressive complex [1]. This PER/CRY complex can translocate into the nucleus and inhibit the suprachiasmatic nucleus proteins CLOCK and BMAL1 from transcription activity resulting in PERIOD and CRYPTOCHROME gene repression [1]. This is called the backward limb of the clock. The forward limb of the clock is when BMAL1 and CLOCK are promoted.
Sample citations:
[6]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
To repeat the citation for other statements, the reference needs to have a names: "Cite error: Closing </ref> missing for <ref> tag. Melatonin increases the magnitude of swarming in cultures of E. aerogenes [8]. However, this pattern is not seen in microbes such as Escherichia coli or Klebsiella pneumoniae [9]. The swarming runs on the 24-hour clock of the circadian rhythm [9]. The MotA promoter on flagellar motor-protein driven plasmids transforms E. aerogenes to express luciferase, revealing circadian patterns of bioluminescence synchronized by melatonin and dependent on temperature [2]. Altogether, these data suggest that the human circadian rhythms may regulate the entrainment of bacterial clocks. Disruption of the circadian clock, either via dietary restriction or jet lag, affects the temporal distribution of the gut microbiome community [5].
Cultured colonies revealed that E. aerogenes proliferated more rapidly in the presence of melatonin than without it [9]. Larger cultures of E. aerogenes exhibited swarming patterns and showed patterns of concentric rings that coincided with the number of incubation days [9]. This showed that the swarming rhythms represent the cycle of a circadian clock [9]. Bioluminescence of E. aerogenes showed a significant effect of melatonin on the phase of peak bioluminescence [9].
The presence of circadian rhythms within symbiotic bacteria in response to endocrine signals regulated by the host’s circadian mechanism adds further credibility to the concept of the microbiota as a metaorganism [9]. One has an intrinsic clock swept by the host’s clock drive signal [9]. Considering our own circadian mechanism as an evolved adaptation to environmental phenomena dominated by a 24-hour cycle, organs and organ systems may be perceived as a takeaway environment for resident microbiota [9]. Thus, environmental perturbations, such as circadian perturbations, affect rhythms within the microbial gut, as previously shown [5].
Gut bacteria possess their own daily rhythmicity in their localization and function. Gut bacteria can modulate host rhythms through microbial metabolites such as butyrate, polyphenolic derivatives, amines, and vitamins.
As mentioned previously, lifestyle stressors such as altered sleep patterns and eating habits can disturb the host circadian cycle and impact the gut microbiome. Several disruptions in the gut can affect substrate oxidation and energy regulation in the host. Disturbances include decreased conjugation of bile acids or increased production of hydrogen sulfide, resulting in a decrease in butyrate production. Sleep patterns and a healthy diet are essential for maintaining gut microbial balance.
The molecular clock that is found in almost every cell contains the genes Per1, Per2, and Per3, the brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1), cryptochrome (CRYPTOCHROME), and the circadian locomotor output cycles kaput gene (Clock). This molecular clock regulated the rhythmic expression of clock-controlled genes. These clock-controlled genes regulate the synthesis, storage, and expenditure of energy.
Section 4
Conclusion
References
- ↑ 1.0 1.1 1.2 2017. Brain basics: Understanding sleep.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Ratiner, K., Elinav, E. “Basic Biology of Rhythms and the Microbiome.” 2017. Circadian Rhythms in Bacteria and Microbiomes 317-328.
- ↑ FitzGerald, G.A. “Timing the microbes: the circadian rhythm of the gut microbiome.” 2017. J Biol Rhythm 32:505-515.
- ↑ Forsyth, C.B., Green, S.J., Engen, P.A., Keshavarzian, A. “Chapter Nine - Circadian Rhythm and the Gut Microbiome.” 2016. International Review of Neurobiology.
- ↑ 5.0 5.1 Wright, J.M., Patel, A.G., Cassone, V.M. "Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. 2016. PloS one.
- ↑ Bartlett et al.: Oncolytic viruses as therapeutic cancer vaccines. Molecular Cancer 2013 12:103.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
