Xylitol in Dental Decay Prevention
Xylitol is a sugar alcohol used as a sugar substitute in food products. Dental decay is the demineralization of the tooth due to the fermentation of carbohydrates by bacteria present in dental plaque, namely Mutans streptococcus. Xylitol is not fermentable by oral bacteria and therefore plays a role in cavity prevention.
Introduction
Dental infections of the teeth and gums are arguably the most common bacterial infections in humans. The mouth contains a diverse population of microbial species. Tooth decay is caused by dental plaque, the accumulation of bacterial communities in aggregates on the tooth surface. Of the 200-300 microbe species indigenous to dental plaque, only a select few are considered dental pathogens which cause tooth decay. Most notable is Mutans streptococcus. It is a cariogenic, i.e. cavity causing, oral pathogen found in dental plaque. It produces extracellular glucans which allow it to form aggregates on the tooth surface. The metabolism of carbohydrates, mainly sucrose, by Mutans streptococcus in the dental plaque produces lactic acid which causes a sharp drop in pH at the tooth surface. This increased acidity causes demineralization beneath the tooth surface which can then lead to the formation of a cavity.[3]
Xylitol is a non-sugar sweetener which is part of the polyols family. It and other sugar alcohols have been used extensively since the 1980s as a sugar substitute in many food products to lower the calorie content and for it’s non-cariogenicity. Xylitol is not metabolized by the bacteria in dental plaque, namely Mutans streptococcus, and therefore does not cause the production of lactic acid and does not contribute to the demineralization which causes dental caries. Xylitol is found primarily in chewing gums. It has been found that chewing gum containing xylitol is effective in preventing dental caries compared with not chewing gum or chewing sugared gum. It is commonly accepted that xylitol is non-cariogenic as well as cariostatic, but it’s anti-cariogenicity properties have yet to be confirmed.[5]
Dental Decay
The tooth surface is a unique environment within the human body because it is both hard and non-shedding. The acquired enamel pellicle is a membranous layer which protects the tooth from bacterial infection. It is formed when the tooth selectively absorbs acidic glycoproteins from the saliva. The acquired enamel pellicle contains a high number of sulfate and carboxyl groups which gives it a net negative charge. Bacteria found in the saliva also have a net negative charge so there is a repulsion between the tooth surface and oral bacteria, protecting the tooth from infection. Mutans streptococcus are not very effective colonizers, although dental plaque does form. [1]
There are many ecosystems on the tooth due to it’s morphology which dictate the bacterial compositions of the plaque. Decay occurs primarily in the molars and premolars. The crown of the tooth, the visible portion of the tooth which is above the gumline, has five different surfaces upon which cariogenic plaque can form. The smooth surfaces on the buccal/labial and lingual sides of the tooth are most prone to plaque formation but will only become decayed in special circumstances such as low saliva conditions or when substrate (carbohydrates) concentrations are high. The front and back of the tooth are also prone to plaque formation but unlike the sides of the tooth, are more prone to decay. In contrast, the chewing surface of the tooth is less prone to plaque formation but is the most prone to caries formation.[1]
Mutans streptococcus are cariogenic streoptococci found in dental plaque which produce extracellular glucans from sucrose that allow it to form aggregates on the tooth surface. There are eight different serotypes, but S. mutans is the most cariogenic. Cavitation is used to measure tooth decay, although cavitation occurs well into the decay process. Dental decay begins with subsurface demineralization followed by a subsurface lesion known as a white spot and finally cavitation. The most caries prone part of the tooth are fissures and contact sites between teeth. These are the parts of the tooth where saliva does not as effectively buffer pH drops resulting from the fermentation of sugars or replenish any mineral lost from the tooth because it is stagnant at that part of the tooth. Saliva is an important defense mechanism against dental decay for it’s buffering abilities. Mutans streptococcus is aciduric, so saliva can effectively inhibit its growth by buffering the environment. Patients exhibiting rampant caries have either frequent or excessive sucrose intake or low saliva flow, and are found to have elevated levels of Mutans streptococcus in their saliva. [1]
Virulence Factors
According to historic and epidemiologic observation, when sucrose is introduced into the diet there is a significant increase in dental decay. Sucrose is a readily fermentable carbohydrate which causes shifts in the populations of plaque flora, namely the increase of Mutans streptococcus. The reverse is also true. When sucrose is restricted, the rate of dental decay and the plaque levels of Mutans streptococcus decreases. When sucrose is in excess there is an increase is lactic acid formation produced by fermentation. Of the sucrose that enters the cell, a small portion is transformed into glucans (glucose homopolymers) by hexosetransferases. These glucans either remain in the cell or diffuse into the environment. In smooth surface decay, glucan formation is a key virulence factor. Glucans act as adhesive material which allows Mutans streptococcus to form adhesive colonies on the tooth surface. It’s ability to form plaque in the presence of sucrose relates to it’s odontopathic activity. [1]
pH and Dental Decay
Following exposure to sucrose or other fermentable carbohydrates, the pH in plaque can drop below 5.0 for anywhere from 30 - 120 minutes. Most plaque bacteria are not metabolically active at such a low pH, therefore S. mutans is selected for in these niches because it is aciduric with a pH optima of 5.0 - 5.5. In times of nutrient excess an intracellular polysaccharide is formed in plaque bacteria including Mutans streptococcus which keeps the plaque ecosystem below pH 5.5 for extended lengths of time after meals, exploiting it’s aciduricity. This is another virulence factor in dental decay correlated with caries activity and carbohydrate consumption. [1]
Antibacterial Properties of Fluoride
Fluoride is a common preventative treatment of dental decay. Fluoride inhibits the metabolic activity in bacteria and the acid pH actually magnifies it’s antimicrobial effect by allowing the uptake of fluoride by the cell more efficient. The aciduricity of Mutans streptococcus is related to the ability of the cell membrane to remain relatively alkaline compared with it’s environment. Sugar is transported into the cell by a proton motive force generated by the pH gradient across the membrane. Fluoride is in the form of HF, which when diffused into the cell at a low pH releases enough hydrogen ions to lower the cytoplasmic pH below the optima of most cytoplasmic enzymes and also dissipates the pH gradient fueling the proton motive force. Fluoride is effective in preventing tooth decay by way of inhibiting the metabolism of cariogenic bacteria, although another approach is to reduce substrate concentrations, effectively starving the cariogenic bacteria and inhibiting fermentation. [1]
Saliva in Decay Prevention
Saliva provides critical defense against dental decay. When food is chewed salivary flow increases which provides a large liquid volume for plaque acids to diffuse into and an increased concentration of bicarbonate buffer which neutralizes acids. Saliva provides a constant remineralization mechanism because it is supersaturated with calcium and phosphate ions. The environment of the tooth surface therefore favors mineral deposition. Additionally, salivary proteins and glycoproteins such as lysozyme, lactoperoxidase, lactoferrin, and high-molecular weight agglutinins, exhibit antibacterial activity although S. mutans is minimally affected by lysozyme. [1]
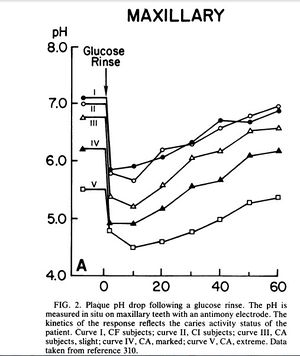
Exposure to fermentable carbohydrates such as sucrose causes a rapid pH drop in dental plaque. A study took pH readings of subjects with varying degrees of decay and then had subjects rinse with a 10% glucose solution. The pH was read at periodic intervals until it returned to it’s original level. The rapid drop in pH shows that glucose was instantaneously converted to acid products, mainly lactic acid, which overwhelmed the buffering capabilities of saliva. In subjects with active dental caries the pH stayed below 5.0 for 20 - 50 minutes while in caries free or caries inactive subjects the pH dropped to around 6.0 and returned to normal after 40 minutes. Further analysis showed that the plaque present at caries active sites produced twice the acid per milligram than the plaque at caries free sites. [1]
Some degree of subsurface demineralization occurs whenever fermentable carbohydrate diffuses into plaque and is converted to acid end-products. Between meals and snacks pH levels return to normal and demineralization is repaired. Remineralization occurs when calcium and phosphate ions in the plaque diffuse into the lesion driven by the supersaturated concentration gradient. When the magnitude and frequency of acid production overwhelms the repair process demineralization leads to cavitation. This can be due to frequent eating or reduction of salivary flow. Remineralization can be encouraged if plaque acid production is restricted through a low sucrose diet, fluoride treatment, or use of sugar substitutes such as xylitol, for snacks in between meals. [1] Chewing gum can also encourage remineralization as it increases saliva flow which has buffering capacity and a high concentration of calcium and phosphate ions which remineralizes dental enamel and resists caries development.
[5]
The Role of Xylitol in Reducing Dental Decay
Sucrose is cariogenic, causing dental decay and eventual cavitation. There are many sugar substitutes which are equally sweet as sucrose but have fewer calories and are non-cariogenic or even anti-cariogenic. Xylitol and sorbitol are two common sugar substitutes that can be used to improve dental health. [6] Xylitol, like other polyols, is a non-cariogenic non-sugar sweetener. Regular consumption of xylitol causes a shift in oral bacteria populations favoring less cariogenic bacteria. [5] The main side effect of polyols is osmotic diarrhea although 4-5 times the amount needed to prevent dental caries would need to be ingested for this to occur. It has been concluded that polyols including xylitol are non-cariogenic. Unlike other sugar alcohols, xylitol has been shown to protect and reduce tooth decay by reducing Mutans streptococcus levels in plaque and saliva and lactic acid production of Mutans streptococcus. One study showed that groups consuming 100% xylitol had greater reduction in caries and S. mutans levels than groups which consumed a combination of xylitol and sorbitol. The amount of xylitol consumed determined the degree of reduction in dental decay, that is, the more xylitol consumed in a day the greater the reduction in dental decay although the benefits of xylitol top out after 10 g per day. Any less than 5 g of xylitol per day is no more effective than sorbitol. It was concluded that other polyol sweeteners may enhance the efficacy of xylitol but will not diminish it. Frequency of ingestion was also found to be important. Any less than 3 doses per day may not be effective. [4]
Mechanism of Action
Unlike sucrose, xylitol has a minimal effect on plaque pH. [4] When plaque is exposed to xylitol concentrations of ammonia and basic amino acids increase which work to neutralize plaque acids. [5] Xylitol is absorbed and accumulates in Mutans streptococcus. It competes with sucrose for cell wall transporters and metabolic processes. The cell expends energy breaking down xylitol with no energy yield and energy-producing intermediates are consumed but not reproduced. This inhibits the growth of Mutans streptococcus. When xylitol is consumed habitually long-term Mutans streptococcus strains are selected for which are less virulent. [4] Xylitol also may affect the adhesive and cohesive properties of plaque which decreases the overall quantity of Mutans streptococcus in dental plaque. Some strains of Mutans streptococcus take up xylitol and convert it to xylitol-5-phosphate which results in the development of intra-cellular vacuoles and degraded cell members. This has a bacteriostatic effect on plaque bacteria. [5]
A study compared the growth of Mutans streptococcus strain OMZ 176 in the presence of xylitol, sorbitol, or glucose in culture media. It was found that 5% of xylitol in the growth medium inhibited growth of Mutans streptococcus compared to media with no extra carbon source added. When xylitol and glucose was added growth was reduced compared with glucose alone. Growth inhibition was dose related. Conversely, sorbitol did not show similar effects. In the presence of xylitol, the dental plaque formed had different physical properties than plaque grown in the presence of sucrose. Plaque grown in the presence of xylitol was less adhesive and hydrated, and also contained less glucan and lipoteichoic acid. [6]
Xylitol Dosing
Xylitol-containing food products are readily available and have great potential to be widely accessible to consumers in order to prevent rampant dental decay. Although, the recommended dosage and usage frequency of xylitol has yet to be concluded. Based on the current research 6 - 10 g divided into at least 3 consumptions per day is necessary to have an effect. Many studies have shown that xylitol-containing gum reduces the extent of dental caries. Candy, toothpaste, tablets, and snack foods are being developed and tested. [4]
Bacteriological studies have shown that the salivary levels of Mutans streptococcus in mothers is related to the initial acquisition in their infants. Once Mutans streptococcus has colonized on childrens’ teeth it’s presence is stable and impairs the establishment of other bacteria on teeth. The earlier the colonization of Mutans streptococcus in children, the higher the caries rates later in life. This study looked at whether mothers chewing xylitol-sweetened gums could help to prevent caries in their children during tooth eruption. After three months, the number of mothers with high Mutans streptococcus levels decreased and showed a decrease in dental plaque. High levels of salivary Mutans streptococcus is a good indicator of caries risk level, so it is a good target for studying the effect of xylitol in caries prevention. The frequency of xylitol consumption was correlated with the level of decrease in salivary Mutans streptococcus levels. A reduction in the mother’s salivary levels significantly affected the levels in her child. The study concluded that xylitol consumption significantly reduced salivary levels in both mothers and children and reduced plaque accumulation on the tooth surface in children. Therefore, children who adopt the habit of regularly chewing xylitol-sweetened gum can reduce caries risk.
[2]
Conclusion
While chewing sugared gum effectively cancels out the benefits of chewing gum on preventing dental decay, chewing gum sweetened with xylitol can have an anticariogenic effect. Xylitol-sweetened gum can be a simple and cost effective solution to dental decay among the near 17 million children in the United States who do not receive basic dental care. The United States Army’s Public Health Command recommends that soldiers and their families chew xylitol-sweetened gum. With further research and support from clinicians, schools potentially have the power to make this recommendation a reality. The dental health of millions of children could be improved if children above the kindergarten age chewed xylitol-sweetened gum 3-5 times per day for just five minutes at a time.
References
Vegarud, Azzev. "Growth Inhibition of Streptococcus Mutans Strain OMZ176 by Xylitol." Acta Pathologica Et Micriobioligica Scandinavica I88.88 (1980): 61-63. Print.
Edited by (Zoe Kiklis), a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.