Yellow Fever Vaccine
Overview
By Christopher Kei Helm
Yellow fever is a human disease caused by the yellow fever virus, prevalent mostly in tropical climates. The yellow fever virus is a part of a family of viruses known as the Flavivirdae and the genus Flavivirus that fall under a broader category of arboviruses, or “arthropod-borne” viruses. In this case, most common arthropod vector of YF is the Aedes aegypti mosquito.
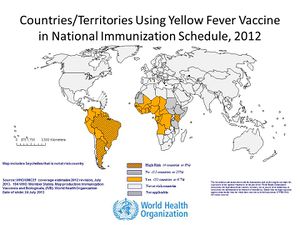
As stated previously, the disease is usually only prevalent in tropical regions of the world, mostly South America and parts of Africa (see figure 2). Even with vaccines available, yellow fever (YF) still causes 30,000 deaths annually with almost 200,000 cases occurring in Africa. Moreover, YF is an acute infection, with mortality rates ranging from 25~50%. Even worse, the number of cases of YF has increased in the past 20 years as a result of declining immunity among susceptible populations to infection, climate change, and urbanization. Because of this, YF is a critical public health issue in South America and Africa.
History
Spaniards documented the first major occurrence of YF in Yucatan, Mexico in 1648. Local Mayans at the time referred to the disease as xekik (black-vomit), which was a common symptom of late stage YF. Previous to arriving in Mexico however, it is hypothesized that the disease originated in Africa and spread as a result of slave ships travelling from Africa and transporting with them infected specimens of A. aegypti in their drinking water.
After the Yucatan outbreak, YF spread even further around the world causing high death tolls even in Barcelona and in the Mississippi Valley. While it is now seen as a foreign tropical disease, there were numerous outbreaks in North America up through most of the 19th century, with epidemics in New York, 4,000 deaths in New Orleans, and even at one point an outbreak in Quebec.
It was not until 1881 when a Cuban scientist, Dr. Carlos Finlay, proposed that YF spread through A. aegypti that people began exterminating the mosquito and subsequently almost completely eradicated YF outside of South America and Africa. The control of the disease was best exemplified by Cuba’s Major William C. Gorgas led and implemented specific mosquito-eradication procedures which effectively eradicated YF in the area within the year in 1901. It was thanks to extermination efforts like Gorgas’ that Panama Canal could even be built considering many workers would have otherwise succumbed to YF as they worked on the canal.
In 1937, Max Theiler created a live-attenuated vaccine for yellow fever by serial-passaging the YF virus through chicken embryotic tissue. This vaccine, known as the 17D vaccine has impressively been highly effective in preventing the disease, with an unusually long antibody persistence upwards of 35 years in some individuals. Since its production, over 500 million individuals have been vaccinated with the 17D vaccine further controlling the spread of yellow fever. Appropriately, Max Thelier was rewarded with a Nobel Prize in 1951 for his contributions.
Virus
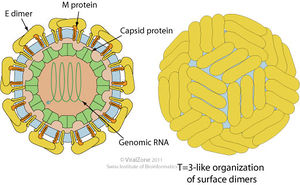
The yellow fever virus (YFV) is a single, positive strand RNA virus with an icosahedral envelope as is characteristic of Flaviviruses and has a diameter of around 50nm. It has a genome of around 10kb. It is further characterized by the mature virions having three major types of structural proteins Capsid (C), Envelope (E), and Membrane (M), which make up the virion envelope. Premature virions on the other hand have M precursor proteins (prM) that form heterodimers with E proteins, making the surface appear spiky. Mature cells maintain a smooth surface as a result of the E proteins forming homodimers in “herringbone-like arrangements.”
Replication
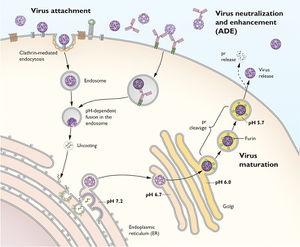
To replicate, the yellow fever virus first uses its E proteins to bind to receptors on the host cell to promote clatharin-mediated endocytosis. Then once the virion reaches the endosome in the eukaryotic cell, the YFV fuses its membrane with the host endosomal membrane and releases the RNA genome into the host’s cytoplasm. Once the single-stranded viral RNA (ssRNA) is released, it is translated and the translated proteins are brought to the endoplasmic reticulum (ER) to be assembled into immature virions. Specifically, the proteins are assembled in certain invaginations in the membrane of the ER called spherules. At these spherules, the ssRNA is also replicated to create new positive sense ssRNA genomes for the new virions.
Once the premature virions are formed, budding off of the ER, they move down the trans-Glogi network and are proteolytically cleaved, breaking down the prM and E heterodimers into M and E proteins to form mature virions. Once mature, the virion particles leave the host cells through secretion.
A large portion of the virion’s ability to replicate relies on the E proteins on its cell surface, which the virion depends on to bind to the host cell’s receptor proteins to enter the host cell as well as to induce fusion of the viral membrane with the host cell. Thus, a significant aspect of neutralizing the virus by the host’s immune system is to produce antibodies that target and disrupt the E protein’s ability to function.