Bacteriophages in Cancer Biology and Treatment
Bacteriophages in Cancer Biology and Treatment
By Salome Shubitidze!
Cancer is the second leading cause of death in the United States. And despite extensive research, billions of dollars in funding, and expert scientists devoting their lives to the search for better therapies, cancer has a way of coming back and claiming lives.More and more studies describe the solid tumor as an extremely difficult cancer to eradicate. There are multiple studies that cite the solid tumor's microenvironment as extremely difficult to infiltrate. Tumors are capable of recruiting the body's own immune cells in order to aid its growth. Bacteriophages are now being considered for cancer therapy due to their incredibly small size, and ability to carry drugs into the tumor microenvironment. Studies have also shown that they are capable of "waking up" the immune system in order to detect tumor cells. This is a new branch of cancer biology and treatment, and has the ability to change the traditional medical treatments of cancer.
The Use of Bacteriophages for Drug Delivery
Chemotherapy drugs, while shown to have anti-tumor effects, tend to result in severe toxicity and widespread distribution throughout the body: notably damaging healthy and malignant cells. New research has started to focus on using bacteriophages as an individualized drug-carrying anti-cancer therapy. The therapy would be targeted, based on genetically-modifying and chemically manipulating filamentous bacteriophages. In Bar et al. 2008, the phages were modified to display a host-specificity-conferring ligand, and carry a cytotoxic drug by chemical conjugationCite error: Closing </ref> missing for <ref> tag Interferons are signaling proteins released by infected host cells that alert nearby cells to strengthen up their defenses.[1]. The continual production of these type of cells, however, leads to chronic inflammation, which has been tied to the spread of cancer. Inflammatory responses occur when damaged cells release chemicals that alert the immune system that something is wrong. White blood cells will flood to the scene, and start the process of creating new cells by dividing existing ones in order to help with rebuilding the damaged tissue. The continual division of cells, leads to a higher likelihood of DNA damage, and subsequently cancer. [2]
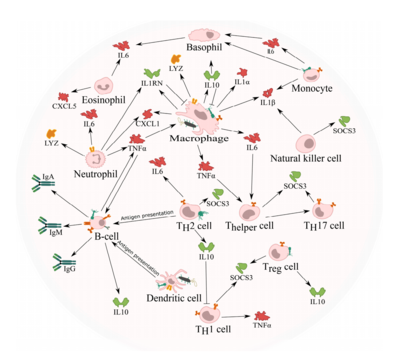
Tumors are particularly clever in their methods of immune evasion. There are many protein factors released by tumor cells that may recruit tumor-associated macrophages (TAMs)[3]. TAMs are a class of immune cells that are primarily found in high numbers in and around the tumor microenvironment. They are responsible for suppressing T-cell mediated anti-tumor immune responses, and are M2-polarized.[4]. M2- polarized macrophages function to repair damaged tissues, and aid the final phase of inflammation repair. The recruitment of TAMS has been found to suppress tumor-specific T cell activation and proliferation. By flooding the the tumor area with TAMS, the tumor is able to trick the body into thinking that repair is needed due to the inflammatory response, instead of actively seeking out the destruction of the cancer cells. Presence of TAMS in and around a tumor microenvironment is correlated with a poor prognosis for those suffering from melanoma or breast cancer specifically. [3] Figure 1. shows more examples of the relationship between tumor growth and the immune system.
Therefore, evidence shows that if our immune system's initial response fails to eradicate tumor cells, the flood of immunoregulatory cells leads to the resurgence of a "smarter" cancer.
Manipulating the Tumor Microenvironment
Recently, bacteriophages have been show to be capable of modifying the tumor environment. Phage coat proteins, and phage DNA tend to elicit strong responses from the innate immune system, prompting phagocytosis and cytokine release.[3]There have been studies that show cytokine storms can be generated as a response to phage infiltration. [5] Tiwari et al. described the need for a neutrophil-phage cooperation in order for the immune system to get rid of P. aeruginosa infections. Therefore, the presence of neutrophils has been demonstrated to be necessary for the eradication of phages. [6]
Delivering phages into the tumor microenvironment has been demonstrated to initiate infiltration of tumor-associated macrophages. Eriksson et al. 2009 found that the microenvironment of bacteriophage-treated tumors switched from M2-polarized to a more M1-polarized environment post phage treatment. Researchers performed gene expression profiling using a LDA made for finding 30 genes preferentially expressed by antigen-presenting cells and encompassing both M1 and M2 markers. Data analysis found that the tumor-specific phage treatment in wild type mice gave way to an increase of inflammatory cytokines such as TNF-, IL-6, and IL-1B.[3]
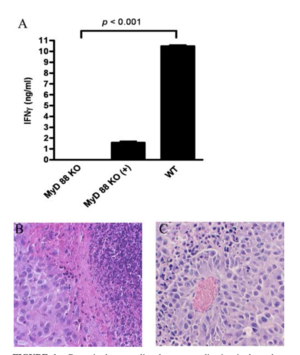
In addition, the study discovered that phage-induced tumor eradication is dependent on Toll-Like receptor (TLR) signaling. Mice that were genetically modified to lack TLR signaling pathways (MyD88 KO mice), were administered a tumor-specific phage treatment. 24 h post treatment, the tumors were looked at histologically. The wild type mice (the ones that had TLR pathways intact) were subject to a massive increase in neutrophils in and around the collected issue, and there was necrosis observed as well. Contrastingly, MyD88 mice had significantly less neutrophil infiltration (see Figure 3). This shows that the immune response that phages evoke are largely carried about by TLR signaling pathways, and that the administration of bacteriophages lead to an increase of neutrophilic granulocytes around the site of cancer.[3]
Bacteriophages are capable of altering the immunosuppressive tumor microenvironment, supporting a M1 environment and the promotion of tumor destruction.[3]
Using Bacteriophages in a Combined Cancer Therapy
Evidence from Gambashidze et al. 2012 has shown that combining E.coli phagelysate and chemotherapy inhibits the rate of cancer growth three fold compared to tumors left untreated. The combined cancer therapy was more potent than chemotherapy and phagelysate administered alone. The study showed that injected doses of phagelysates did not lead to malignant growth, and was accepted in mice.[7]
Studies have shown that the tumor microenvironment is altered once phages are administered.[3] Other cancer treatments have been shown to not be as effective because of the complications that accompany the solid tumor microenvironment. The area itself has little to no circulation, and even is considered hypoxic due to the constant cell growth. This makes it difficult for therapies such as chemotherapy, radiation, and hypothermia magnetic nanoparticle treatment to reach and destroy cells deep within the tumor. [8] This is why the combining of several cancer treatments has gained some interest over the last few years.
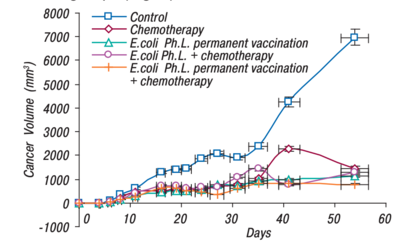
Gambashidze et al. 2012 compared the efficacy of using E. coli phagelysate and chemotherapy as a combined treatment, with each treatment on its own(see Figure 1). As shown, all forms of cancer treatment did greatly reduce the tumor's volume, but the combined treatment of E.coli phagelysate and chemotherapy had the lowest volume of all the tested treatment types.
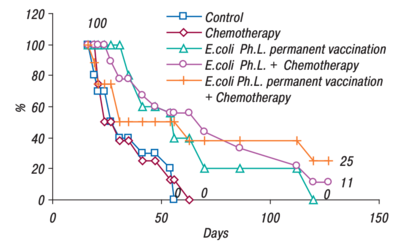
An efficacy study was also performed by Gambashidze's team(see Figure 2). The combined treatment group lived the longest, 25% of mice making it to 130 days. Compared to the control and chemotherapy groups, where all the mice were deceased just after day fifty. All the groups that had phagelysate treatment, lived significantly longer than their counterparts(figure 2).[7]
Conclusion
There are many novel applications of bacteriophage treatment that can be added to the toolbox of an oncologist. While this is a relatively new therapy, it has lots of potential. The use of phages as a drug delivery system has shown promise, as it allows for targeted delivery of chemotherapy drugs. Chemotherapy drugs traditionally lack specificity, and consequently result in widespread damage of healthy cells in addition to cancerous ones. By using a targeted therapy, physicians can avoid long-term immune and cell damage. Bacteriophages have also been shown to manipulate the tumor microenvironment, a task that has proven difficult for many other novel and traditional therapies. By activating the immune system, the phages can alert the body to the tumor. The combination of therapies has shown great effectiveness in mice studies. By changing the tumor microenvironment, other cancer therapies have an advantage towards destroying tumor cells that are wound tightly within the hypoxic area. While there is still much left to be discovered with regard to the effects of phage treatment, these studies provide evidence that this therapy is directed towards success.
References
- ↑ https://jvi.asm.org/content/84/13/6549 Erin L. Lousberg, Cara K. Fraser, Michael G. Tovey, Kerrilyn R. Diener, John D. Hayball Journal of Virology Jun 2010, 84 (13) 6549-6563; DOI: 10.1128/JVI.02618-09
- ↑ [https://www.cancer.gov/about-cancer/causes-prevention/risk/chronic-inflammation National Cancer Institute "Chronic Inflammation" April 2015.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 [https://www.ncbi.nlm.nih.gov/pubmed/19234207 Eriksson, Fredrik & Tsagozis, Panagiotis & Lundberg, Kajsa & Parsa, Roham & Mangsbo, Sara & Persson, Mats & Harris, Robert & Pisa, Pavel. (2009). Tumor-Specific Bacteriophages Induce Tumor Destruction through Activation of Tumor-Associated Macrophages. Journal of immunology (Baltimore, Md. : 1950). 182. 3105-11. 10.4049/ jimmunol.0800224.
- ↑ [https://jhoonline.biomedcentral.com/articles/10.1186/s13045-019-0760-3#citeas Lin, Y., Xu, J. & Lan, H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 12, 76 (2019). https://doi.org/10.1186/s13045-019-0760-3
- ↑ 5.0 5.1 Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2019, 11, 10.
- ↑ [Tiwari, B.R.; Kim, S.; Rahman, M.; Kim, J. Antibacterial efficacy of lytic Pseudomonas bacteriophage in normal and neutropenic mice models. J. Microbiol. 2011, 49, 994–999]
- ↑ 7.0 7.1 7.2 7.3 [http://dspace.nbuv.gov.ua/bitstream/handle/123456789/139076/11-Gambashidze.pdf?sequence=1 Gambashidze, Ketevan & Khorava, P. & Azaladze, T. & Azaladze, A. & Lasareishvili, Besarion & Jaiani, Ekaterine & Tediashvili, Marina. (2012). ANTI-TUMOR EFFECTS OF PHAGELYSATES OF E.COLI. 16-16.
- ↑ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3689267/ Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904‐5912. doi:10.1038/onc.2008.271
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.