Vax1 Homeobox Genes and Mammalian Embryo Development
Introduction
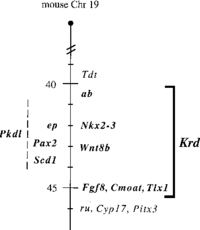
Vax1 is a novel homeobox gene discovered 1998 by a group of researchers in Max Planck Institute for Biophysical Chemistry[1] in the human and mouse genome, crucial to the development of the vertebrate forebrain, olfactory, and visual systems of mammals. Vax1 directly regulates the activation of transcriptional factors such as Emx2, which is a protein coding gene that is crucial in the synthesis of the neural and ciliary structure of the frontal systems of the roof and archipallium of the brain during early development. Through the use of interspecific backcross analysis in mice, crossing a hybrid with one of its parents or an individual genetically similar to its parent, the Vax1 genome was accurately mapped on the distal regions of the mouse chromosomal region, located on mouse chromosome 19. Therefore, Vax1 is directly linked to the homeobox genes, Pax2, Fgf8, MxiL, Emx2, and Aop1, which are located on the same chromosome 19. [2] [3] The development of the forebrain and optic nerves due to mutations of Vax1 homeobox gene, would cause phenotypical impairments in mammals. These include delay in neurological development, decrease in the eye/optic nerve size, and labial malformations.[4]
Classification, Structure, and function
The Vax1 homeobox gene is a transcription factor that is classified in the Emx/Not genome family, and located specifically on the mouse chromosome 19 and human chromosome 10, in the nucleus of somatic/germline cells. Vax1 is highly specific in function with the ability of initiating DNA Binding and activation of linked transcription factors such as Vax2 and Emx2(both are in the same genome family as Vax1), inducing biological processes such as cell differentiation, proliferation, organization, and complete system development.[5]
In further detail, the Vax1 genome is significant in the expression of several phenotypic traits of an organism, primarily focusing on the cardiovascular, olfactory systemm and forebrain in the organism. For instance, it was discovered that mice with a specific mutation in the Vax genome labeled Vax1tm1b(KOMP)Mbp tend to experience the phenotypic trait of abnormal sinus arrhythmia, altering the pace in which the heart beats, and ultimately changing the pace in which the creature inhales and exhales.
Mutation
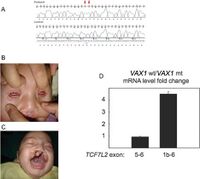
Mutations in the Vax1 homeobox gene in the human chromosome 10, such as successive nucleotide substitutions c.453G>A and c.454C>A, could lead to drastic malformations of the olfactory system of the body, causing an array of detrimental phenotypic characteristics. This includes genetic diseases such as anophtalmia and microphthalmia. In the published paper lead by Anne M. Slacorinek 2011, are they able to have the first case study in researching the phenotypic impact Vax1 homeobox genes has on a human organisms, rather then mice. With the use of an active case study, a male offspring who suffers from a successive nucleotide addition mutation, inhibits the proper function of the Vax1 genome leading to physical impairments such as orofacial clefting, and micropthalmia. [6]
In addition to physical impairments in result of point mutations in the Vax1 genome, because of the innactivity of the Vax1 genome, there is major stunted growth of the forebrain, leading to developmental cognition impairments. When the human patient was reviewed at the age of 3.5 years old, it was determined his developmental level was equivalent to the level of a 6 month old child. [7]
Conclusion
section.
References
- ↑ Hallonet M;Hollemann T;Wehr R;Jenkins NA;Copeland NG;Pieler T;Gruss P; “Vax1 Is a Novel Homeobox-Containing Gene Expressed in the Developing Anterior Ventral Forebrain.” Development (Cambridge, England). U.S. National Library of Medicine. Accessed November 7, 2021. https://pubmed.ncbi.nlm.nih.gov/9636075/.
- ↑ Wikimedia Foundation. (2021, July 2). Backcrossing. Wikipedia. Retrieved November 7, 2021, from https://en.wikipedia.org/wiki/Backcrossing.
- ↑ Hallonet, M., Hollemann, T., Wehr, R., Jenkins, N. A., Copeland, N. G., Pieler, T., & Gruss, P. (1998). Vax1 is a novel homeobox-containing gene expressed in the developing anterior ventral forebrain. Development, 125(14), 2599–2610. https://doi.org/10.1242/dev.125.14.2599
- ↑ Slavotinek, A. M., Chao, R., Vacik, T., Yahyavi, M., Abouzeid, H., Bardakjian, T., Schneider, A., Shaw, G., Sherr, E. H., Lemke, G., Youssef, M., & Schorderet, D. F. (2011). Vax1 mutation associated with microphthalmia, corpus callosum agenesis, and orofacial clefting: The first description of a VAX1 phenotype in humans. Human Mutation, 33(2), 364–368. https://doi.org/10.1002/humu.21658
- ↑ VAX1 MGI mouse gene detail - MGI:1277163 - ventral anterior homeobox 1. (n.d.). Retrieved November 7, 2021, from http://www.informatics.jax.org/marker/MGI:1277163.
- ↑ Slavotinek, A. M., Chao, R., Vacik, T., Yahyavi, M., Abouzeid, H., Bardakjian, T., Schneider, A., Shaw, G., Sherr, E. H., Lemke, G., Youssef, M., & Schorderet, D. F. (2011). Vax1 mutation associated with microphthalmia, corpus callosum agenesis, and orofacial clefting: The first description of a VAX1 phenotype in humans. Human Mutation, 33(2), 364–368. https://doi.org/10.1002/humu.21658
- ↑ Slavotinek, A. M., Chao, R., Vacik, T., Yahyavi, M., Abouzeid, H., Bardakjian, T., Schneider, A., Shaw, G., Sherr, E. H., Lemke, G., Youssef, M., & Schorderet, D. F. (2011). Vax1 mutation associated with microphthalmia, corpus callosum agenesis, and orofacial clefting: The first description of a VAX1 phenotype in humans. Human Mutation, 33(2), 364–368. https://doi.org/10.1002/humu.21658
Edited by Logan Gusmano, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2021, Kenyon College.