Toxoplasma gondii: Mode of Infection and Effect on Neurological Cells
By [Alexander S. McQuiston]
Introduction
Toxoplasma gondii is one of the most common cyst parasites around the world due to its ability to infect almost all warm blooded organisms, including humans (Gaskell et al. 2009). Some estimates of T. gondii infections are reported to be as high as a third of the world’s population (Tenter et al. 2000; Weiss et al. 2009). More specifically, T. gondii is an intracellular heteroxenous parasite belonging to the apicomplexan phylum. The apicomplexan phylum is a large group of parasitic protists that contain specialized organelles required for target host invasion starting at the apical pole (Dubey et al. 1998; Weiss 2011)). Infection of T. gondii causes toxoplasmosis in both healthy and immunocompromised organisms. When the parasite infects healthy organisms the disease is normally asymptomatic, but if the parasite infects an immunocompromised organism, such as an AIDS patient, diseases like encephalitis can occur (Carruthers et al. 2007). Along with medical diseases, T. gondii has also been hypothesized to cause a variety of neurological disorders such as schizophrenia (Weiss et al. 2009).
T. gondii has both intermediate and definitive hosts. The intermediate hosts include a wide variety of warm blooded animals from rodents to humans, but only has one definitive host, felines (cats) (Tenter et al. 2010). T. gondii is also capable of both asexual and sexual reproduction. When the parasite is in an intermediate host it reproduces asexually, and when the parasite is in its definitive host it is able to reproduce sexually. The ultimate goal of T. gondii is to infect its definitive host so it can sexually reproduce and then go on to infect other organisms (Webster et al. 2007). An interesting way T. gondii has been able to facilitate its transmission from an intermediate host to its definitive host is by invading target host cells and then manipulating host cellular behavior and physiological processes (Prandovsky et al. 2011; Webster et al. 2007).
Life Stages
There are three main infectious stages, all intracellular, of T. gondii: tachyzoites, bradyzoites (cyst forming), and sporozoites. Both tachyzoites and bradyzoites asexually multiply while oocysts sexually multiply (Dubey et al. 1998).
Tachyzoites
Tachyzoites are responsible for the acute infection by rapidly multiplying inside a host which causes the overall population of the parasite to grow. It can also be considered the more aggressive parasite stage because this stage of the parasite moves around the body and invades target host cells (Carruthers et al. 2007). Sporozoites go through differentiation into tachyzoites once the infection is ready to take place. Tachyzoites will multiply within a cell until cell lysis and the new parasites can move on to infect more target host cells. Tachyzoites are mainly found within intestine epithelial cells (Dubey at al. 1998).
Bradyzoites
Bradyzoites are considered to be slower metabolic and reproductive parasites. Following the invasion by tachyzoites, tachyzoites can go through differentiation in to bradyzoites if certain environmental factors are met such as a decrease in reproductive success or if the tachyzoite can no longer manipulate host cellular processes (Kamerkar et al. 2012). Once differentiated in to bradyzoites and the formation of a cyst wall forms around the bradyzoites, the bradyzoites slowly divide and fill up the cyst. Cysts are mainly found in neurological tissue and muscle tissues. Bradyzoites were originally thought to be static structures that caused chronic toxoplasmosis, but recent studies have shown that they play a role in behavioral manipulation and also break down host cells in order to invade new cells (Dubey et al. 1998).
Sporozoites
Sporozoites are the only T. gondii stage that sexual reproduce. When bradyzoites reach a definitive host (a cat), they differentiate into sporozoites. Sporozoites are found within oocysts that reside in the intestinal tract of cats (Tenter et al. 2000). Sporozoites are expelled from the definitive host in fecal matter. This stage of T. gondii is spore-like and can survive outside of the definitive host in soil and water for about a year. Sporozoites contribute to infection of intermediate hosts via ingestion (Dubey et al. 1998).
How T. gondii Infects Host Cells
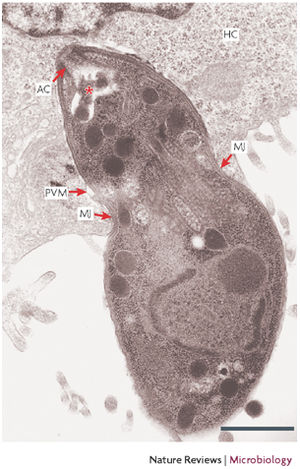
T. gondii infect warm blooded animals by 3 main pathways (in order of most to least common): horizontally transferred via ingestion of the parasite when eating undercooked meat from livestock that was infect prior to slaughter, horizontally transferred via the ingestion of oocyst that can be found in water and soil that has been contaminated with cat fecal matter, and vertically transferred from mother to fetus during pregnancy (Carruthers et al. 2007; Tenter et al. 2000). Once T. gondii infects its host, it must be able to create a balance between altering cellular processes in order to produce ideal conditions while not causing damage that would render an immune response.
Most often, T. gondii is ingested in the form of oocyst or a tissue cyst containing bradyzoites (Carruthers et al. 2007; Tenter et al. 2000). Once inside the gut of the host organism, proteolytic enzymes breaks down the proteins that the cyst wall is made of, releasing the sporozoites found within the oocyst or tissue cyst (Werk et al. 1985). The sporozoites act as free parasites that are able to move through the intestinal epithelial, differentiate into tachyzoites, and invade epithelial cells (Gopal et al. 2008).
The Invasion
There are two ways in which parasites are able to invade hosts: phagocytosis or active invasion. Active invasion requires energy from the parasite while phagocytosis does not require energy from the parasite and occurs when the host cell engulfs the parasite. T. gondii is thought to go through active invasion based on the time it takes for the parasite to get inside the host cell and with the involvement of the host during the invasion process. The total time elapsed between first contact by the parasite and the final invasion of the parasite is between 15 and 40 seconds, a much more rapid event than phagocytosis and the host cell uses energy to produce extracellular structures (Werk et al. 1985).
Before invasion begins, T. gondii must find a target cell and specifically orient itself in relation to the target. The invasion is initiated by the apical pole of T. gondii coming in to contact with the target cell. The apical pole of T. gondii includes a conoid and rhoptries. A conoid is a cone shaped organelle that is thought to play a major role in the initial penetration of the parasite. The conoid creates an indentation in the membrane of the host cell which causes the host to produce extracellular protrusions called pseudopods that stabilizes the connection between the host cell and the apical pole of parasite (Werk et al. 1985). The production of extracellular pseudopods is considered active invasion because the host cell’s protrusion production requires energy. As the conoid indents the host cell’s membrane and the host cell’s pseudopods stabilize the host-parasite connection site, rhoptries secrete specialized proteins, called penetration enhancing factors (PEFs), which decreases the viscosity of the host’s membrane (Werk et al. 1985). By decreasing the viscosity allows for easier penetration and rupturing of the host cell’s membrane. PEFs function has been observed to be determined by low concentrations of ions. For example, a high concentration of ions such as Ca2+ decreases the fluidity of cellular membranes which ultimately decreases host cell viscosity making invasion more difficult. The rhoptries also secrete proteins that contribute to the development of host cell extracellular protrusions and a vacuole around the parasite (Werk et al. 1985).
The newly formed vacuole is known as a parasitophorous vacuole that consists of both host cell membrane lipids and rhoptry content. Vacuole formation begins when the parasite makes about a 1.5 μm “cut” in the host cell’s membrane that the parasite uses to enter the cell (Hirai et al. 1966). Microcinematography has previously displayed a constricting pattern along the length of the parasite as it entered through the opening (Hirai et al. 1966). As the parasite enters the host, the parasite is able to extend its conoid and apical pole inside the host cell. As the parasite becomes medially constricted, the extracellular protrusions are then hypothesized to aid in pulling the opposite pole into the cell (Werk et al. 1985).
T. gondii has also been previously observed to move during the invasion. The movement has been described as a form of drilling or spinning (Hirai et al. 1966). Drilling or spinning motions have the ability to increase the rate at which the parasite invades the host cell. The invagination made by rhoptries and the conoid in the host cell’s membrane is extremely small and decreases invasion efficiency. A drilling or spinning movement by the parasite could increase the size of the original invagination and make the final steps of invasion easier (Werk et al. 1985).
Inhibiting Invasion
There are not many ways in which T. gondii invasion is able to be inhibited. One way, stated earlier, is the concentration of ions such as Ca2+ (Werk et al. 1980). As the concentration increases, the fluidity of a target host cell’s membrane can decrease making invasion more difficult. Another factor that has been observed to reduce invasion rate of T. gondii is the presence of cytochalasin. Cytochalasin is a metabolite that is able to block the production of long actin filaments. The extracellular protrusions produced by the host cell thought to aid in invasion are composed of actin filaments. Disrupting the ability of the host cell to produce actin filaments would not allow the host cell to aid in invasion (Werk et al. 1985).
Infecting Neurological Tissue
As T. gondii sporozoites enter the gastrointestinal tract they differentiate in to tachyzoites. The tachyzoites travel around the intestinal epithelial invading epithelial cells, before targeting specialized cells that are circulated (macrophages) throughout the body to reach neurological tissues such as the brain (Carruthers et al. 2007). Invading macrophages to travel around the body provides the tachyzoites with protection from any immune responses. As the tachyzoites travel around the body they eventually reach the brain where they invade neurological cells such as neurons, astrocytes, and glial cells (Carruthers et al. 2007). Once inside these neurological cells and after facing stresses, tachyzoites go through differentiation to become bradyzoites. Differentiation has been hypothesized to a result of an inability to manipulate a host’s cell cycle or there is a decrease in nutrients needed to multiply rapidly (Kamerkar et al. 2012). The parasitophorous vacuole that is used to protect the tachyzoite within the host cell differentiates in to a cyst wall that surrounds the newly formed bradyzoites creating an intracellular tissue cyst. Tissue cysts are the ultimate reason for chronic infection of T. gondii. These cysts that were once thought to be a dormant, static phase of T. gondii that was used to simply infect other host organisms, has been found to lead to the infection of new neurological cells and play a major role in host behavioral changes (Weiss et al. 2011).
T. gondii's Effect on the Brain
Most warm blooded animals, including humans are intermediate hosts of T. gondii, meaning that the parasite can only reproduce asexually when infecting that host. The definitive hosts of T. gondii are felines (cats) in which they can reproduce sexually. Along with T. gondii's ability to evade intermediate host immune systems, it also has a unique ability to manipulate intermediate host behavior when infecting neurological cells (Webster et al. 2013). Previous studies have shown that T. gondii causes behavioral changes in rodents such as mice and rats. Mice and rats are genetically programmed to dislike the odor produced by cats which allows them to determine when a cat is near-by and avoid predation. When rodents are infected with T. gondii they lose their dislike or aversion to the cat’s odors and are attracted to them instead. This leads to increased predation and increased transfer of T. gondii to its definitive host (Webster et al. 2007). The mechanism responsible for T. gondii behavioral change in rodents is still unknown to scientists.
Rodents are not the only intermediate organisms that have been observed to experience behavioral changes when infected with T. gondii. T. gondii infected humans have also been observed to experience behavioral changes and neurological disorders (Flegr et al. 2007). One neurological disorder that has a direct correlation with infection is schizophrenia (Webster et al. 2013). Schizophrenia is a lifelong disease that affects about 1% of the population. Although schizophrenia is associated with genetics and a wide range of environmental factors, T. gondii puts people at a greater risk than genetics and other environmental factors. The exact cause of schizophrenia is unknown, but one possibility is an imbalance of chemicals in the brain, specifically an increase in dopamine. In a recent study, T. gondii infected mice were observed to have increased dopamine metabolism than mice not infected (Prandovszky et al. 2011).
One hypothesis for T. gondii’s ability to increase dopamine is the presence of genes that encode for an enzyme that aids in dopamine metabolism. An example of this kind of enzyme would be tyrosine hydroxylase. The main function tyrosine hydroxylase is to catalyze the rate limiting step of dopamine metabolism, the synthesis of L-3,4-dihydroxyphenylalanine (L-DOPA) from the L-tyrosine amino acid. The production of L-DOPA is important in the dopamine metabolism because it is the precursor for the dopamine. An increase in enzymatic productivity of L-DOPA would lead to an increased dopamine metabolic rate and ultimately an imbalance within the brain (Prandovszky et al. 2011).
T. gondii infected hosts have been observed to have highest levels of cyst formations in the amygdala and nucleus accumbens. These two regions of the brain have also been observed to contain levels of dopamine up to 114% higher when cells are infected compared to uninfected (Stibbs et al. 1985). Dopamine found in these regions plays an important function in reward to stimuli, motivation, pleasure, and fear. The level of dopamine found in these regions is regulated, but the introduction of T. gondii has the ability to increase dopamine levels and alter the way our brain reacts to stimuli. T. gondii will increase tyrosine hydroxylase-like enzymatic activity that can produce the precursors for dopamine metabolism. High amounts of dopamine observed in vitro studies suggest that dopamine is being properly transferred, but it does not reflect the amount of dopamine that would be observed in vivo. Tissue cysts in vivo contain many more parasites than in vitro tissue cysts, suggesting that in vivo infections could result in a much higher amount of dopamine released (Prandovszky et al. 2011).
Encysted parasites can intake molecules needed for the production of L-DOPA from a channel in the parasitophorous vacuole which would also allow for the release of L-DOPA into the cytosol of neurological host cells where dopamine is produced. Dopamine produced in neurons are packaged in to vesicles and then transported to axons, so when extra dopamine is produced by cysts and released into the cytosol of neurons it is also packaged and transported along axons (Prandovszky et al. 2011). This creates a flooding of dopamine, especially if there are many cysts within neurons.
Tyrosine Hydroxylase
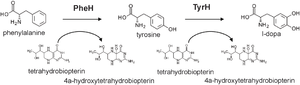
Gaskell et al. (2009) analyzed T. gondii genome in order to observe any enzymes that could play a role in dopamine catalysis. Two regions of genes within the genome displayed high similarity to each other as well as the catalytic domain found in mammalian hydroxylases. Interestingly, T. gondii’s genome suggests the presence of two hydroxylases, TgAaaH1 and TgAaaH2, which display catabolic activity in the presence of tyrosine and phenylalanine. Although able to catabolize both, the enzymes displayed preference for tyrosine when in the presence of both tyrosine and phenylalanine suggesting that the enzyme acts more like a tyrosine hydroxylase, but is still considered bi-functional.
Although the genome of T. gondii codes for both of these tyrosine hydroxylases and therefore are expressed in all phases of T. gondii, the amount of expression of each enzyme depends on the phase that the parasite is in at that specific time. There is also different expression between the parasite strains (McConkey et al. 2013). T. gondii strains have been separated into Type I, Type II, and Type III based on differences in genetic make-up. Variation in genetic make-up causes each strain to have a different effect in the host cells that it infects. Type I strain T. gondii are virulent and have the ability to infect both healthy and immunocompromised organisms causing acute toxoplasmosis, the type II strain is the main strain obtained from immunocompromised organisms such as AIDS patients, and the type III is generally avirulent. Type I strains are mostly found as tachyzoites due to aggressive infection and type III strains consist mostly of bradyzoites and are less active (Carruthers et al. 2007). The differences in infection effects coupled with the differences in tyrosine hydroxylases suggests that there is a correlation between which the highest regulated enzyme and the resulting infection effects. For example, there is greater induction of TgAaaH2 and bradyzoite specific genes found in Type III strain than in Type I strain. This ultimately suggests that T. gondii increases hydroxylase expression during cyst formation which occurs in neurological tissues and cells (Gaskell et al. 2009).
The increased expression of tyrosine hydroxylase in bradyzoites and type III strains because this stage of the T. gondii life cycle is consistent with a slow metabolic rate (Gaskell et al. 2009). This could be a way for the parasites to avoid host immune responses while still physiologically altering cellular behavior and processes. Tachyzoites multiply rapidly and draw a lot of attention from the host immune system from lysis of the host cell and the strong presence of foreign bodies. Bradyzoites are in a cyst form and up until recently have been thought to be a dormant stage allowing them to go unnoticed by the immune system (Weiss et al. 2011). If tachyzoites were the main source of tyrosine hydroxylase activity then there would not be as much of a prolonged and significant effect on host behavior as there would be if bradyzoites performed the most enzymatic activity.
Both T. gondii tyrosine hydroxylases have a C terminal domain and an N terminal domain. The C-terminal domain is responsible for catalysis and the N-terminal domain plays a role in substrate specificity. The regulation process of activity is not fully understood. It is hypothesized to be a complex regulatory system because of similarities the T. gondii tyrosine hydroxylase has with eukaryotic hydroxylases (Gaskell et al. 2009). For example, the T. gondii enzyme’s N-terminal domain has an extension that has the potential for phosphorylation which is also found in eukaryotic tyrosine hydroxylases. Although the regulation of enzymatic activity is unknown, T. gondii tyrosine hydroxylase depends on the co-factor biopterin that aids in catalysis. How the parasitic tyrosine hydroxylase gains access to biopterin is not fully understood (Gaskell et al. 2009). There are few enzymes that are biopterin dependent and the source of biopterin that the dependent enzymes use is unknown. Once in the presence of biopterin and tyrosine hydroxylase is activated, both enzymes preferentially L-DOPA from tyrosine which is the rate limiting step in dopamine metabolism. L-DOPA must enter the host cell cytosol in order to complete the synthesis of dopamine. Fluorescence has shown that the parasite’s tyrosine hydroxylase is located near the edges of the cell. The location of the enzyme suggests that the enzyme and L-DOPA can be secreted outside of the cell through channels or secretory proteins to complete the synthesis of dopamine (McConkey et al. 2013). Ultimately, it is hypothesized that T. gondii encoded tyrosine hydroxylases are responsible for the increase of dopamine levels.
Conclusion
Toxoplasma gondii has three different life stages that all play a crucial role in the infection and pathology of toxoplasmosis. The different stages have different roles that allow for maturation and increase of parasite population within a host to make the infection most successful. Tachyzoites, bradyzoites, and sporozoites also have the ability to alter host cell behavior and physiological process. The ability to affect rodent behavior has been extensively studied, but T. gondii may also affect human behavior by inducing neurological disorders such as schizophrenia. A leading hypothesis behind T. gondii causing schizophrenia is the disruption in dopamine levels that are a direct result of tyrosine hydroxylase enzymes expressed by bradyzoites in neurological cells. Further experimentation will need to be performed in order to deduce the mechanisms behind T. gondii induced manipulation of cellular behaviors and physiological processes.
References
1. Carruthers, V.B., T. Suzuki. 2007. Effects of Toxoplasma gondii infection on the brain. Schizophrenia Bulletin. 33(3): 745-751.[1]
2. Dubey J.P., D.S. Lindsay, C.S. Speer. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clinical Microbiology Reviews. 11(2): 267-299.[2]
3. Flegr, Jaroslav. 2007. Effects of Toxoplasma on human behavior. Schizophrenia Bulletin. 33(3): 757-760.[3]
4. Gaskell, E., J.E. Smith, J.W. Pinney, D.R. Westhead, G.A. McConkey. 2009. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS ONE. 4(3): e4801. Doi:10.1371/journal.pone.0004801 [4]
5. Gopal, R., D. Birdsell, F.P. Monroy. 2008. Regulation of toll like receptors in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol. 30(11-12): 563-576.[5]
6. Hirai, K., K. Hirato, R. Yanagawa. 1966. A cinematographic study of the penetration of cultured cells by Toxoplasma gondii. Jpn J Vet Res. 14: 81-90.[6]
7. Kamerkar, S., P.H. Davis. 2012. Toxoplasma on the brain: understanding host-pathogen interactions in chronic CNS infection. J. Parasitology Research. 2012: 1-10.[7]
8. McConkey, G.A., H.L. Martin, G.C. Bristow, J.P. Webster. 2013. Toxoplasma gondii infection and behavior – location, location, location? J. Exp. Biology. 216: 113-119.[8]
9. Prandovszky, E., E. Gaskell, H. martin, J.P. Dubey, J.P. Webster, G.A. McConkey. 2011. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE. 6(9): e23866. Doi:10.1371/journal.pone.0023866
10. Stibbs, H.H. 1985. Change in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 79: 153-157.
11. Tenter A.M., A.R. Heckeroth, L.M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int J Parasitol. 30(12-13): 1217-1258.
12. Webster, Joanne P. 2007. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophrenia Bulletin. 33(3): 752-756.
13. Webster, J.P., M. Kaushik, G.C. Bristow, G.A. McConkey. 2013. Toxoplasma gondii infection, from predation to schizophrenia: can animal behavior help us understand human behavior? J. Exp. Biology. 216: 99-112.
14. Weiss L.M., J.P. Dubey. 2009. Toxoplasmosis: a history of clinical observations. Int J Parasitol. 39(8): 895-901.
15. Weiss, L.M., K. Kim. 2011. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 5: D391-D405.
16. Werk, R., W. Bommer. 1980. Toxoplasma gondii: membrane properties of active energy-dependent invasion of host cells. Tropenmed Parasitol. 31: 417-420.
17. Werk, R. 1985. How does Toxoplasma gondii enter host cells? Reviews of Infectious Diseases. 7(4): 449-457.
