Engineered bacterial symbionts of Anopheles gambiae as inhibitors of Plasmodium falciparum
Introduction
By Nontokozo Mdluli
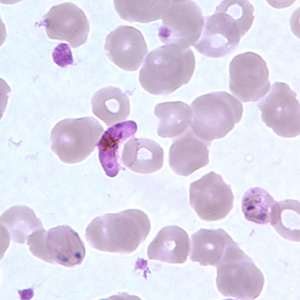
Malaria is known to be the world’s deadliest parasitic disease, with over 800 million people contracting the disease annually. Malaria is the main cause of death in the tropical regions with at least a million people dying annually from contracting the disease [1]. The disease is transmitted by vector mosquitoes such as the female Anopheles gambiae mosquito. The causative agent of malaria that is transferred from vector mosquitoes to hosts is the parasite Plasmodium, of the five known species of the parasite Plasmodium falciparum is the deadliest (Figure 1) [2].
In Africa, the mosquito Anopheles gambiae remains the most widely distributed Plasmodium falciparum vector in the region, responsible for millions of deaths every year. Thus, malaria vaccines continue to be developed to find new mechanisms to control the disease. The current prescribed malaria vaccine involves immunization with an attenuated Plasmodium falciparum strain in its sporozoite form [2]. The initial stages of malaria vaccine development involved studies on Plasmodium berghei whose natural hosts are rodents. The rodents were immunized with X-ray irradiated, attenuated Plasmodium berghei sporozoites obtained from the salivary glands of infected mosquitoes [2]. The researchers discovered that immunized mice with the radiation attenuated parasite Plasmodium berghei developed immunity in later infections with the Plasmodium parasite. The development of immunity to later infections was an indication that a plausible target for inhibiting the complete transmission of the parasite Plasmodium in the mosquito vector had been discovered. The challenge since then has been developing a highly effective vaccine that directly targets the parasite in either the host cells or the mosquito vector.
In the past two decades, malaria vaccine development has expanded to target the three different stages of Plasmodium development; (1) the initial stage of mosquito vector to host transmission, which is the stage of liver cell infection; (2) the erythrocytic stage which is the phase at which the host red blood cells are infected with the Plasmodium parasite; (3) the sexual stage, which is the phase at which the mosquito has taken up the parasite, and Plasmodium sexually reproduces midgut of the vector [2]. The most successful vaccine candidates thus far have been the ones that target the pre-erythrocytic phase of parasitic development, the phase of initial transfer of the parasite from mosquito to human. The vaccine RTS,S has been the most developed and investigated vaccine candidate so far. The vaccine aims to target the pre-erythrocytic stage of development of the Plasmodium parasite when the parasite sporozoites are still in the circulatory system, right before they invade the liver hepatocytic cell. The vaccine targets the Plasmodium CS protein which induces antibodies that respond to infection by sporozoites [3]. The RTS,S vaccine is currently in phase III testing in 5 -7 sub-Saharan African countries where malaria is endemic. Because the Plasmodium life cycle is so complex and because development of the parasite is so time sensitive, there have been a lot of challenges with developing the RTS,S vaccine, and many other vaccines [2].
Other more successful vaccines have conferred immunization with DNA vectors to induce antibodies against sporozoites and to inhibit the development of the parasite at different stages of differentiation. Another combination of treatments aim to target the growth rate of the Plasmodium parasite in the sexual replication stage of development. Still, studies in malaria control continue to accelerate, focusing on better ways to deliver antigens in the mosquito vector in order to induce immunological responses to inhibit the parasite development in the mosquito vector [3].
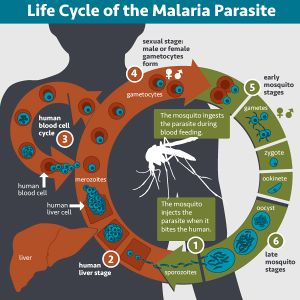
Bacterial symbionts are abundant in a lot of organisms including insects that are disease vectors, such as mosquitos and flies. Recent studies have tried to determine the mechanisms of these symbiotic relationships with the intentions of developing ways to intervene vector borne diseases such as malaria [1]. During transmission of sporozoites from infected host to vector mosquito, thousands of gametocytes may be ingested by the mosquito vector. These gametocytes later develop into gametes which fuse to produce zygotes. Successful fertilization of the gametes results to production of ookinetes. The ookinetes then proceed to invade and penetrate the epithelial cells lining the midgut of the mosquito vector where they develop into oocysts. When oocysts mature, they rupture releasing sporozoites which travel to the mosquito salivary glands waiting for the next transmission from the mosquito vector to a host [1]. The abundance of midgut microbiota in the mosquito vector creates a midgut bottleneck. With a large microbiota population colonizing the midgut of the mosquito, this compartment becomes a highly competitive environment for symbiotic bacteria as well as pathogenic bacteria [1].
Research has shown that the microbiota bottleneck present in the midgut of the mosquito vector has a severe impact on the development of Plasmodium oocysts, such that even if a mosquito vector ingests a large number of gametocytes, only a handful eventually develop into oocysts [4]. This interference in oocyst development in the midgut of the mosquito vector indicates that Plasmodium transmission success may be determined by microbiota composition [4]; if Plasmodium transmission can be effected by microbiota composition in the midgut of the mosquito vector, how can the mosquito midgut be genetically modified to express effector genes that inhibit Plasmodium development [1]? Paratransgenesis has been expanded in malaria vaccine development and treatment, by using genetically modified symbionts of the mosquito vector to produce effector molecules that interfere with Plasmodium development midgut of the mosquito vector [5]. This crucial midgut relationship between the mosquito vector and microbiota has prompted further studies in paratransgenesis in malaria vaccine development and treatment.
Sample citations: [1]
[2]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Plasmodium falciparum Life cycle
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
Plasmodium falciparum development in Anopheles gambiae midgut
Include some current research, with at least one figure showing data.
Microbial Symbiosis of Anopheles gambiae
Include some current research, with at least one figure showing data.
Symbiotic Control (SC) of Anopheles gambiae
Conclusion
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2016, Kenyon College.
