Porphyromonas gingivalis
Ron Ramsay, Bench C, September 23, 2016 [3]
Classification
Higher order taxa
(domain) Bacteria — (phylum) Bacteroidetes — (class) Bacteroidia — (order) Bacteroidales — (family) Porphyromonadaceae — (genus) Porphyromonas [4][5]
Species
Porphyromonas gingivalis
Type strain: 2561 = ATCC 33277 = BCRC 14417 = CCRC 14417 = CCUG 25893 = CCUG 25928 = CIP 103683 = Coykendall 2561 = DSM 20709 = JCM 12257 = KCTC 5121 = NCTC 11834 = Slots 2561 [5][6]
Description and significance
[2] P. gingivalis bacteria are Gram-negative, obligately anaerobic, non-motile, and non-sporeforming. Morphology can be short rod-shaped, or in broth culture, coccobacilli 0.5 µm by 1-2 µm in size. On solid media, colonies are generally smooth, shiny and convex. On blood agar medium, they darken within a week due to the take-up of heme which is oxidized into hemin which accumulates on the cell surface. This characteristic colony colour which we describe as black is how the genus name Porphyromonas was derived, from a Greek word for "purple".
A virulent strain of P. gingivalis—W83—was first isolated in the 1950s by H. Werner in Germany from an undocumented human oral infection.[7][8] In 2003, W83 became the first strain of P. gingivalis to be sequenced.[9]
In humans, P. gingivalis is most associated with the subgingiva of the oral cavity where it forms part of the biofilm that is dental plaque, causing the painful inflammation of periodontal disease, eventually leading to tooth loss.[10] Periodontal disease affects 10–15% of adult worldwide.[11]
Genome structure
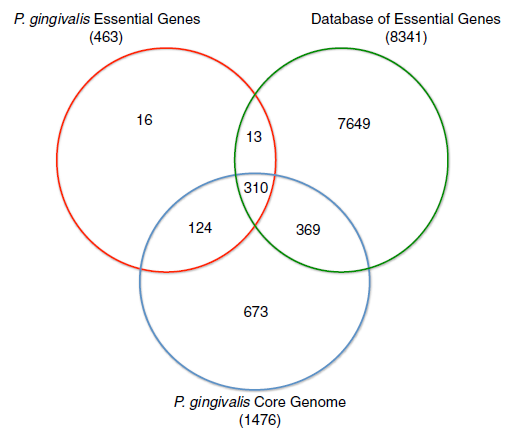
The whole genome sequence of P. givivalis type strain ATCC 33227 was determined in 2008 by Naito et al.[13], deposited as Genbank accession AP009380.[14] The genome comprises a single circular chromosome is 2.35 million bp in size, averaging 48.4% G+C content, and is without a plasmid. Coding sequence coverage is 86.1%. The 2090 protein coding sequences identified have an average length of 970 bp.[13]
Mutagenesis experiments have identified 463 genes essential for in vitro viability for strain ATCC 33227, and of those, 424 are contained within its core genome of 1476 genes.[12]
According to the "mouse subcutaneous soft tissue abscess model", AATC 33227 is classified as a "less virulent" strain. [13] This property lends itself to comparative studies against the "more virulent" strains in order to identify their virulence factors. Naito et al. performed a genomic comparison analysis between the AATC 33227 and the "more virulent" W83 strain.[13] Both genomes contain 53 tRNA genes and 4 rRNA operons. Various types of mobile elements are present in both strains, including insertion sequences, miniature inverted-repeat transposable elements (MITEs) and conjugative transposons. Sequence alignment shows that extensive rearrangements have taken place between the two strains to produce strain-specific coding sequences (CDSs) 60% specific to ATCC 33277, 68% specific to W83. Gene similarities to other bacterial species such as Bacteroides fragilis suggests that horizontal transfers have taken place.
Cell structure and metabolism
The Gram-positive P. gingivalis has an outer capsule which varies in chemical composition and thickness between strains. The capsule seems to serve two main roles. Firstly, the capsule may be necessary to facilitate attachment to other cells within a biofilm.[15] Secondly, a thicker capsule can reduce phagocytosis by leukocytes.[16]
The fimbriae of P. gingivalis are the major determinants of adhesion, and are well characterized.[10] There are 3 types, which are are up to 3 µm long and 5 nm wide.
P. gingivalis is non-motile, anaerobic and asaccharolytic (i.e. unable to catabolise carbohydrates). Gingipains are a group of cystein endoproteases peculiar to P. gingivalis on which it is highly dependent to gain nutrients from host cell stubrates.Cite error: Closing </ref> missing for <ref> tag
</references>
This page is written by Ron Ramsay for the MICR3004 course, Semester 2, 2016
- ↑ 1.0 1.1 Nakayama, K. (2015). Porphyromonas Gingivalis and Related Bacteria: From Colonial Pigmentation to the Type Ix Secretion System and Gliding Motility. J Periodontal Res 50:1-8. doi:10.1111/jre.12255.
- ↑ 2.0 2.1 2.2 Shah, H. N. and M. D. Collins (1988). Proposal for Reclassification of Bacteroides Asaccharolyticus, Bacteroides Gingivalis, and Bacteroides Endodontalis in a New Genus, Porphyromonas. Int J Syst Evol Microbiol 38:128-31. doi:10.1099/00207713-38-1-128.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMICR3004 - ↑ 4.0 4.1 NCBI Taxonomy Browser
- ↑ 5.0 5.1 5.2 StrainInfo.net. Porphyromonas gingivalis
- ↑ 6.0 6.1 LPSN: List of prokaryotic names with standing in nomenclature. Porphyromonas
- ↑ 7.0 7.1 Loos, B. G., D. W. Dyer, T. S. Whittam, and R. K. Selander (1993). Genetic Structure of Populations of Porphyromonas Gingivalis Associated with Periodontitis and Other Oral Infections. Infection and Immunity 61:204-12. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC302706/
- ↑ 8.0 8.1 Ménard, C. and C. Mouton (1995). Clonal Diversity of the Taxon Porphyromonas Gingivalis Assessed by Random Amplified Polymorphic DNA Fingerprinting. Infection and Immunity 63:2522-31. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC173337/
- ↑ 9.0 9.1 Nelson, K. E., R. D. Fleischmann, R. T. DeBoy, I. T. Paulsen, D. E. Fouts, J. A. Eisen, S. C. Daugherty, R. J. Dodson, A. S. Durkin, M. Gwinn, D. H. Haft, J. F. Kolonay, W. C. Nelson, T. Mason, L. Tallon, J. Gray, D. Granger, H. Tettelin, H. Dong, J. L. Galvin, M. J. Duncan, F. E. Dewhirst, and C. M. Fraser (2003). Complete Genome Sequence of the Oral Pathogenic Bacterium Porphyromonas Gingivalis Strain W83. J Bacteriol 185:5591-601. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC193775/
- ↑ 10.0 10.1 10.2 Socransky, S. S. and A. D. Haffajee (2002). Dental Biofilms: Difficult Therapeutic Targets. Periodontol 2000 28:12-55. http://onlinelibrary.wiley.com/doi/10.1034/j.1600-0757.2002.280102.x/abstract
- ↑ 11.0 11.1 , P. E. and H. Ogawa (2012). The Global Burden of Periodontal Disease: Towards Integration with Chronic Disease Prevention and Control. Periodontol 2000 60:15-39. doi:10.1111/j.1600-0757.2011.00425.x.
- ↑ 12.0 12.1 12.2 Klein, B. A., E. L. Tenorio, D. W. Lazinski, A. Camilli, M. J. Duncan, and L. T. Hu (2012). Identification of Essential Genes of the Periodontal Pathogen Porphyromonas Gingivalis. BMC Genomics 13:578. doi:10.1186/1471-2164-13-578.
- ↑ 13.0 13.1 13.2 13.3 13.4 Naito, M., H. Hirakawa, A. Yamashita, N. Ohara, M. Shoji, H. Yukitake, K. Nakayama, H. Toh, F. Yoshimura, S. Kuhara, M. Hattori, T. Hayashi, and K. Nakayama (2008). Determination of the Genome Sequence of Porphyromonas Gingivalis Strain Atcc 33277 and Genomic Comparison with Strain W83 Revealed Extensive Genome Rearrangements in P. Gingivalis. DNA Res 15:215-25. doi:10.1093/dnares/dsn013.
- ↑ 14.0 14.1 Genbank nucleotide sequence database. accession: AP009380.1: "Porphyromonas gingivalis ATCC 33277 DNA, complete genome"
- ↑ 15.0 15.1 Rosen, G. and M. N. Sela (2006). Coaggregation of Porphyromonas Gingivalis and Fusobacterium Nucleatum Pk 1594 Is Mediated by Capsular Polysaccharide and Lipopolysaccharide. FEMS Microbiol Lett 256:304-10. doi:10.1111/j.1574-6968.2006.00131.x.
- ↑ 16.0 16.1 Katz, J., D. C. Ward, and S. M. Michalek (1996). Effect of Host Responses on the Pathogenicity of Strains of Porphyromonas Gingivalis. Oral Microbiol Immunol 11:309-18. http://www.ncbi.nlm.nih.gov/pubmed/9028256
- ↑ Bakaletz, L. O. (2004). Developing Animal Models for Polymicrobial Diseases. Nat Rev Micro 2:552-68. http://dx.doi.org/10.1038/nrmicro928
- ↑ How, K. Y., K. P. Song, and K. G. Chan (2016). Porphyromonas Gingivalis: An Overview of Periodontopathic Pathogen Below the Gum Line. Front Microbiol 7:53. doi:10.3389/fmicb.2016.00053.
- ↑ Sbordone, L. and C. Bortolaia (2003). Oral Microbial Biofilms and Plaque-Related Diseases: Microbial Communities and Their Role in the Shift from Oral Health to Disease. Clin Oral Investig 7:181-88. doi:10.1007/s00784-003-0236-1.
- ↑ Slots, J., J. J. Kamma, and C. Sugar (2003). The Herpesvirus-Porphyromonas Gingivalis-Periodontitis Axis. J Periodontal Res 38:318-23. http://onlinelibrary.wiley.com/doi/10.1034/j.1600-0765.2003.00659.x/abstract
- ↑ Tanner, A. C. R. (2015). Anaerobic Culture to Detect Periodontal and Caries Pathogens. J Oral Biosci 57:18-26. doi:10.1016/j.job.2014.08.001.
- ↑ Veillard, F., B. Potempa, Y. Guo, M. Ksiazek, M. N. Sztukowska, J. A. Houston, L. Koneru, K. A. Nguyen, and J. Potempa (2015). Purification and Characterisation of Recombinant His-Tagged Rgpb Gingipain from Porphymonas Gingivalis. Biol Chem 396:377-84. doi:10.1515/hsz-2014-0304.

