Oral Microbiome and Cognitive Decline
Introduction to the Oral Microbiome
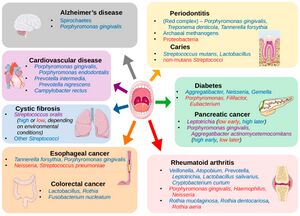
The oral microbiome is a dynamic and intricate ecosystem of microorganisms inhabiting the oral cavity, playing a pivotal role in maintaining oral and overall health [1]. It comprises a diverse array of bacteria, fungi, viruses, and protozoa, with more than 700 bacterial species and 1,000 phylotypes identified to date [2] [3]. Similar to fingerprints, the oral microbiome exhibits substantial inter-individual variability, rendering it a unique and distinctive feature of each person [4].
The oral microbiome's composition and function are influenced by various factors, including genetics, diet, oral hygiene, and environmental exposures [3]. Bacterial species in the oral cavity can be broadly categorized into three groups: commensals, opportunistic pathogens, and pathogens [1]. Commensal bacteria are generally harmless and can even confer health benefits, while opportunistic pathogens may cause disease under certain conditions. In contrast, pathogens are consistently associated with disease states [2].
Recent research has emphasized the importance of the oral microbiome in human health, revealing its connection to various systemic conditions such as cardiovascular diseases, diabetes, and even cognitive function [5] [2]. (Figure 1) Among the hundreds of bacterial species in the oral microbiome, some well-known representatives include Streptococcus mutans, associated with dental caries, and Porphyromonas gingivalis, linked to periodontal disease [3]. Other notable bacteria include the nitrogen-fixing Fusobacterium nucleatum, which plays a critical role in the development of dental plaque, and the highly diverse Actinomyces species, involved in the formation of dental biofilms [2].
The balance between pathogenic and commensal bacteria is essential for maintaining oral health, and disruptions in this equilibrium can lead to oral infections and other complications [1]. For instance, dysbiosis, an imbalance in the microbial community, can result in the overgrowth of pathogenic species, contributing to periodontitis and other oral diseases [5].
Advances in molecular techniques, such as oligotyping, have allowed scientists to explore the human oral microbiome at a higher resolution, uncovering its complex structure and interactions [4]. This deeper understanding has led to the development of novel strategies for promoting oral health, as well as the prevention and treatment of oral diseases [2].
The relationship between the oral microbiome and cognitive function is an emerging area of research. While a direct link is yet to be firmly established, there is evidence suggesting that chronic oral infections, such as periodontitis, can contribute to systemic inflammation and the release of pro-inflammatory cytokines, which may in turn affect cognitive function [5]. Furthermore, some studies have identified specific oral pathogens, such as Porphyromonas gingivalis, in the brains of Alzheimer's disease patients, implying a potential connection between the oral microbiome and neurodegenerative disorders [2]. These findings highlight the need for further investigation to better understand the interplay between oral health, the microbiome, and overall well-being.
Alzheimer's Disease Overview
Alzheimer's disease (AD) is the most common form of dementia, affecting millions of people worldwide and posing significant public health challenges [6]. It is a progressive neurodegenerative disorder characterized by cognitive decline, memory loss, and impaired daily functioning [7]. The exact cause of AD remains unclear, but it is believed to involve a complex interplay of genetic, environmental, and lifestyle factors [8].
At the cellular level, two hallmark features of AD are the presence of extracellular amyloid plaques and intracellular neurofibrillary tangles [7]. Amyloid plaques are primarily composed of aggregated amyloid-β (Aβ) peptides, which result from the cleavage of amyloid precursor protein (APP) by β- and γ-secretases [8]. The accumulation of Aβ peptides is thought to play a critical role in the pathogenesis of AD, although the exact mechanisms by which they contribute to neurodegeneration remain under investigation [6].
Increasing evidence suggests that bacteria may play a role in the development of AD. One hypothesis is that certain bacterial infections, particularly those involving the oral cavity, can induce systemic inflammation and the production of pro-inflammatory cytokines, which may contribute to neuroinflammation and the accumulation of amyloid plaques [8]. In fact, some studies have identified specific oral pathogens, such as Porphyromonas gingivalis, in the brains of AD patients, supporting the potential link between oral microbiome dysbiosis and AD [7].
Given the significant public health burden of AD, understanding the potential role of bacteria and other factors in its pathogenesis is critical for developing effective prevention and treatment strategies [6]. Further research is needed to elucidate the complex interplay between the oral microbiome, systemic inflammation, and neurodegenerative processes in AD and other cognitive disorders.
Amyloid Plaque and Neurofibrillary Tangles
Amyloid plaques and neurofibrillary tangles are two hallmark pathological features of Alzheimer's disease, a neurodegenerative disorder characterized by progressive memory loss and cognitive decline. Both amyloid plaques and neurofibrillary tangles have been associated with synaptic dysfunction, neuronal loss, and impaired brain function (Gómez-Isla et al., 1997; Guillozet et al., 2003).
Amyloid plaques are extracellular deposits composed primarily of β-amyloid (Aβ) peptides, which are derived from the proteolytic cleavage of the amyloid precursor protein (APP). The aggregation of Aβ peptides into insoluble fibrils leads to the formation of amyloid plaques (Gouras et al., 2015). These plaques can disrupt synaptic communication, leading to neuronal dysfunction and ultimately cell death (Mathis et al., 2004). In addition, activated microglia and astrocytes, which are glial cells involved in the brain's immune response, have been shown to surround amyloid plaques, contributing to the inflammatory response in Alzheimer's disease (Nagele et al., 2004).
Neurofibrillary tangles, on the other hand, are intracellular aggregates of hyperphosphorylated tau protein. Tau is a microtubule-associated protein that helps maintain the stability of microtubules in neuronal axons. In Alzheimer's disease, abnormal hyperphosphorylation of tau leads to the formation of paired helical filaments, which aggregate into neurofibrillary tangles (Guillozet et al., 2003). These tangles disrupt the neuronal cytoskeleton, impairing axonal transport and ultimately leading to neuronal death (Gómez-Isla et al., 1997).
While amyloid plaques and neurofibrillary tangles are characteristic of Alzheimer's disease, they can also be found in the brains of cognitively normal older adults, suggesting a complex relationship with the natural aging process (Guillozet et al., 2003). It is important to note that the presence of these pathological features does not necessarily lead to clinical symptoms, as other factors, such as synaptic resilience and compensatory mechanisms, can influence cognitive function in the aging brain (Mathis et al., 2004).
Recent evidence has suggested that oral bacteria, particularly those associated with periodontal disease, can contribute to the development of amyloid plaques and neurofibrillary tangles in Alzheimer's disease. For instance, Porphyromonas gingivalis, a keystone pathogen in periodontitis, has been detected in the brains of Alzheimer's patients, and its toxic proteases (gingipains) have been found to co-localize with tau and Aβ (Dominy et al., 2019). This suggests a possible link between oral bacteria and the pathological features of Alzheimer's disease.
In summary, amyloid plaques and neurofibrillary tangles are two key pathological features in Alzheimer's disease, which have been associated with neuronal loss and impaired brain function. These features can also be found in the brains of cognitively normal older adults, highlighting the complex relationship with the natural aging process. Oral bacteria, particularly those related to periodontal disease, may contribute to the development of these pathological features, emphasizing the importance of oral health in maintaining cognitive function throughout aging.
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ 1.0 1.1 1.2 [Wade, William G. “The Oral Microbiome in Health and Disease.” Pharmacological Research, SI:Human microbiome and health, 69, no. 1 (March 1, 2013): 137–43. https://doi.org/10.1016/j.phrs.2012.11.006. ]
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 [Verma, Digvijay, Pankaj Kumar Garg, and Ashok Kumar Dubey. “Insights into the Human Oral Microbiome.” Archives of Microbiology 200, no. 4 (May 1, 2018): 525–40. https://doi.org/10.1007/s00203-018-1505-3.]
- ↑ 3.0 3.1 3.2 [Deo, Priya Nimish, and Revati Deshmukh. “Oral Microbiome: Unveiling the Fundamentals.” Journal of Oral and Maxillofacial Pathology : JOMFP 23, no. 1 (2019): 122–28. https://doi.org/10.4103/jomfp.JOMFP_304_18. ]
- ↑ 4.0 4.1 [Deo, Priya Nimish, and Revati Deshmukh. “Oral Microbiome: Unveiling the Fundamentals.” Journal of Oral and Maxillofacial Pathology : JOMFP 23, no. 1 (2019): 122–28. https://doi.org/10.4103/jomfp.JOMFP_304_18.]
- ↑ 5.0 5.1 5.2 [Jenkinson, Howard F. “Beyond the Oral Microbiome.” Environmental Microbiology 13, no. 12 (2011): 3077–87. https://doi.org/10.1111/j.1462-2920.2011.02573.x.]
- ↑ 6.0 6.1 6.2 [Ballard, Clive, Serge Gauthier, Anne Corbett, Carol Brayne, Dag Aarsland, and Emma Jones. “Alzheimer’s Disease.” The Lancet 377, no. 9770 (March 19, 2011): 1019–31. https://doi.org/10.1016/S0140-6736(10)61349-9.]
- ↑ 7.0 7.1 7.2 [Scheltens, Philip, et al. "Alzheimer's disease." The Lancet 388.10043 (2016): 505-517 https://doi.org/10.1016/S0140-6736(15)01124-1]
- ↑ 8.0 8.1 8.2 [Querfurth, Henry W., and Frank M. LaFerla. “Alzheimer’s Disease.” New England Journal of Medicine 362, no. 4 (January 28, 2010): 329–44. https://doi.org/10.1056/NEJMra0909142.]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College
