Nitrogen Cycle
Introduction
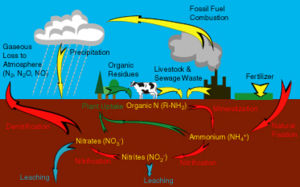
The nitrogen cycle is an important chemical cycle which occurs in the ecosystem. The nitrogen cycle begins with nitrogen fixation. Organic nitrogen is then transformed by organisms to NH4. This process is known as mineralization the conversion of organic nitrogen into inorganic nitrogen. This NH4 is than converted into nitrite which is than transformed into nitrate which is the process of nitrifcation. Nitrification is carried out mainly by nitrifying bacteria such as nitrococcus, and nitrobacter. Nitrification occurs when the environment is aerobic. When the environment is anaerobic the nitrate undergoes denitrification which can have severe consequences with regards to the atmophere. Denitrification is when nitrate gets converted into atmospheric nitrogen which is a greenhouse gas. Immobilization is another step in this cycle and is the conversion of nitrate to organic nitrogen. The nitrogen cycle is important because it results in important compounds being produced which are essential for proper growth of plants and other organisms.
Nitrogen cycle processes

Nitrogen is an essential nutrient for all life on earth. It is present in various forms such as dinitrogen gas, organic nitrogen, and ammonium and nitrate ions. Microbially mediated processes transform nitrogen form one form to another. These transformations include nitrogen fixation (transformation of dinitrogen to ammonia), ammonification/ mineralization (conversion of organic nitrogen to ammonium), nitrification (conversion of ammonium to nitrate; occurs in aerobic conditions), immobilization (assimilation), and denitrification (reduction of nitrate to gaseous oxide and dinitrogen; occurs in anaerobic conditions)
Nitrogen fixation
Atmospheric nitrogen (N2) is abundant, but unavailable for biological activity due to the high energy required to break the triple bond. In nitrogen fixation, atmospheric nitrogen is reduced to biologically useful ammonia (NH3) by the activity of prokaryotes utilizing nitrogenase. Species such as Azotobacter, Bradyrhizobium and Rhizobium have the ability to fix nitrogen. Azotobacter are free living organisms, while Bradyrhizobium and rhizobium live mutually with plants.
Chemistry
The reaction catalyzed by nitrogenase is N2 + 8H+ + 8e- ---> 2NH3 + H2. Ammonia produced in this way is rapidly protonated to NH4+. Nitrogenases are irreversibly inhibited by exposure to O2, and thus organisms must take steps to prevent this. Some organisms, such as Azotobacter protect their nitrogenases with a thick capsule that slows oxygen diffusion. Others, such as the cyanobacteria Nostoc form special cells with thick cell walls to exclude oxygen, allowing the cell to provide fixed nitrogen to its neighbors. Alternative nitrogenases do exist that are not deactivated by oxygen, but they are found in a relatively small number of organisms.
Key microorganisms
Nitrogen fixed by prokaryotes constitutes the vast majority of all biologically active nitrogen on the planet. Diazotrophs is the category of all organisms that are capable of fixing nitrogen (Sylvia et al., 2005). Nitrogen-fixers may be aerobic or anaerobic, and may be free-living or in a symbiotic relationship with a plant. One type of symbiotic bacteria, now collectively known as rhizobia, are found in root nodules on legumes. They use the constant source of carbon from the legumes to be able to fix nitrogen. There are other symbiotic relationships including: Ferns and cyanobacteria, Fungi and cyanobacteria, and Frankia (an actinomycete) which can be in a relationship with a variety of plants (Sylvia et al., 2005). Free living diazotrophs, like Azotobacter, are able to live in an array of environments because of their independence from plants.
Nitrogen Mineralization
Nitrogen mineralization is the sum of concurrent ammonium production and consumption processes. It is sometimes used in a generic sense for the production of inorganic nitrogen, both ammonium and nitrate (Sylvia et al, 2005).
Ammonification or gross nitrogen mineralization is the conversion of organic-nitrogen compounds to ammonium. This process is mediated by heterotrophic microbes. Production of ammonium involves several steps. First organic nitrogen is broken down by extracellular enzyme. Then the resulting products pass across cell membrane and are metabolized, resulting in ammonium production, which release into the soil solution.
Naturally, ammonification and nitrogen immobilization are coincidence. There are several factors that influence whether there is net production or consumption by microorganisms in soil. The general principle is that net production occurs when nitrogen is not limiting.
Mineralization generally occur at C/N ratios < 20 (Scow 2008).
Key microorganisms
The microbial degradation of amino compound and nucleic acids is driven by the consumption of heterotrophic microbes for carbon and energy. Thus the ammonium released as a result of ammonification can be considered a by-product of catabolism(Sylvia et al., 2005).
Nitrogen Immobilization
Immobilization is the conversion of ammonium and nitrate to organic nitrogen, primarily as a result of the assimilation of ammonium by the microbial biomass.The process requires energy for the conversion of nitrate to ammonium and subsequent incorporation of ammonium into amino acids.
Key microorganisms
Heterotrophic microbes and other organisms assimilate ammonium to build up biomass.
C/N Ratios
A carbon to nitrogen ration is the ratio of the mass of organic carbon to the mass of total nitrogen in soil or organic matter (Sylvia et al. 2005) C/N ratios dictate whether the nitrogen gets immobilized or mineralized. If the C/N ratio is less than 20 than the nitrogen will get mineralized. At C/N ratios >30 the process of immobilization will occur (Scow 2008).
If organisms have an excess of nitrogen than they will excrete NH4+ back into the soil, returning nitrogen back into the system (Scow 2008). If they are nitrogen depleted they will take nitrogen out from the soil leading to nitrogen deficiencies in the soil.
Nitrification
Nitrification is the microbial production of nitrate from the oxidation of reduced nitrogen compound. This process is two-step process. The first step of lithoautotrophic nitrification is ammonia oxidation, the conversion of ammonium to nitrite by ammonia-oxidizing bacteria of Nitroso- genera. Then nitrite is oxidized to nitrate by the nitrite-oxidizing bacteria of the Nitro- genera (Sylvia et al., 2005). The process of nitrication is an acidifying reaction, and approximately 1-3% of the nitrogen is lost as a gas in the form of NO/N2O during nitrification (Dahlgren, 2007). The nitrate produced can be lost from the soil ecosystem in two ways: through denitrification (concern for global warming), or through leaching(concern for groundwater management).
Ammonia Oxidation: NH3 + 1.5 O2 --> NO2- + H++H2O
Nitrite Oxidation: NO2- + H2O --> NO3- + 2H+ + 2e-
Key microorganisms
Bacteria involved- Nitrosomonas, Nitrobacter, e.g. Nitrobacter hamburgensis, Nitrobacter winogradskyi, Nitrococcus mobilis,and archaea are key players in this processes. As an example of their contribution; Archaea oxidize ammonia in temperatures of 80 degrees C and pH levels of 2 to 4.5 in some hot springs of Iceland. (Richter 2006)
Denitrification
Denitrification is the conversion of nitrate to nitrogen gas in presence of low oxygen (Sylvia et al., 2005). Nitrates are reduced to nitrites, and then the reduction of nitrites to nitrogen gas occurs. Because this process is carried out under anoxic or near anaerobic conditions, to create this condition in the experiment, soils were flooded by water. Denitirfication represents an overall loss of Nitrogen from a soil ecosystem, as nitrate is converted to an atmospheric form of nitrogen. The overall or reaction is:
2 NO3- + 5H2 + 2H+ --> N2 + 6 H2O
The process of denitrification releases nitrous oxides into the atmosphere which is a problem regarding global warming. Nitrous oxide is a greenhouse gas and is a compound responsible for the warming of the earth.
Key microorganisms
Most microorganisms who mediate this process are facultative anaerobic organoheterotrophs. Thiomicrospira is a group of sulfur oxidizing bacteria. They are distributed among the gamma and epsilon subdivisions of the Proteobacteria. Thiomicrospira denitrificans is a species who has ability to utilize nitrate as electron acceptor in denitrification.
Specific enzymes (Nar, Nir, Nor, Nos) are involved in the process of denitrification. Redox reactions Nar- nitrate to nitrite,Nir- nitrite to nitrogen monoxide,Nor- nitrogen monoxide to nitrous oxide, Nir- nitrous oxide to atmospheric nitrogen.These enzymes may be in other forms of nitrogen bacteria, but the gene that controls the different enzymes may be turned off or on.
Ecological Significance
Nitrogen is a vital nutrient in the environment and is used by plants and other organisms to maintain proper growth and cellular function (Proteins, Nucleic Acids). Nitrogen is often regarded as being the limiting nutrient in ecosystems because it is required by organisms and is usually in short supply. Steps in the nitrogen cycle offers a way to return nitrogen to the system. Nitrogen is returned to the system by nitrification and mineralization while it is lost though denitrification and leaching.
Bacteria play a significant role in the nitrogen cycle since they convert atmospheric nitrogen into a form which can be utilized by other organisms such as plants. Because the ammonium cation is adsorbed more strongly to soil particles than the nitrate anion, nitrate can flow with water which can be taken up through the roots.
Nitrogen is important to micro organisms because certain microbes such as facultative anaerobes use nitrate as terminal electron acceptors. This is especially important in the process of denitrification, the conversion of nitrate to atmospheric nitrogen (NO/N2).
However nitrogen can also cause a lot of problems for ecosystems and people. The Science Daily released an article "Changing Global Nitrogen Cycle Impacting Human Health, Says Colorado University-led Study"; this article states that the changes in the global Nitrogen Cycle causes health issues in humans. The health issues include "respiratory ailments, heart disease and several cancers." (sciencedaily.com)
The Nitrogen Cycle, Greenhouse gases and Groundwater pollution
The process of denitrification releases nitrous oxide into the atmosphere. Nitrous oxide or "laughing gas" is the form of nitrogen that is a green house gas. Along with carbon dioxide, ozone, methane, water vapor, and halocarbons, nitrous oxide causes the blanket of air surrounding the earth to thicken; thus warming up the earth itself. This phenomenon has been dubbed "Global Warming" and understanding it's causes is key to preventing the mass destruction that will happen if green house gases remain unchecked. (The Environmental Literacy Council)
Denitrification can help to mitigate nitrate levels associated with ground water pollution . Nitrate is a very mobile form of nitrogen and has been responsible for ground water pollution. Its mobility is due to its negative charge which repels it from clay particles in the soil. Nitrate is readily leached from the environment and can lead to ground water pollution. Nitrate pollution is a major concern and a cause of "blue baby syndrome" seen in infants. Denitrification reduces the amount of nitrate from the environment by converting it into atmospheric nitrogen which is a greenhouse gas.
Because nitrate can be used as an electron acceptor by denitrifying bacteria, the addition of an electron donor such as sodium formate or hydrogen may help in the degredation of nitrate.
Current Research
The Center of Limnology is currently studying the rise in organic nitrogen availability to microbes. Their findings may lead to an analysis of the consequences of anthropogenic nitrogen enrichment watersheds. To learn more visit their website at http://cires.colorado.edu/limnology/research/
The nitrogen fertilizers cause environmental problems associated with leeching into our water systems and production of greenhouse gas. To decrease the usage of the fertilizers,the alternative ways to increase nitrogen available to plants are investigated. Nodulation process forming of symbiosis between nitrogen-fixation bacteria and plant is one of the interested research nowadays. Recent research has shown the evidences that the genes, called symbiosis-receptor-kinase-gene (SMYRK), are involved in a genetic program that links arbuscular mycorrhiza (AM) and one form of bacterial nodule symbiosis. Moreover, the analysis of SYMRK in several species of plant provided the evidence that the functions of SYMRK constituted an important step in the evolution of intracellular nodule symbiosis. Most plants have a short version of SMYRK, which is required for AM symbiosis. A longer variant of SMYRK was found only in plants involved in the symbiotic relationships with nitrogen-fixing bacteria. Importantly, the longer version was found in both legumes which form symbioses with rhizobia and in actinorhiza which form symbiotic relationships with Frankia bacteria, about which there is little genetic information. The results therefore suggest a common evolutionary origin of intracellular root symbioses with nitrogen-fixing bacteria (Markmann et al., 2008).
Another interesting research find regarding the nitrogen cycle is that the application of road salts can severely disrupt the nitrogen cycle(Green et al., 2008). There is evidence to suggest that sodium reduces the cation exchange capacity of ammonium reducing the ammonium concentration from the soil (Green et al., 2008).
References
Sylvia, D.M., Fuhrmann, J.J., Hartel, P.G.,& Zuberer, D.A. (2005). Principles and application of soil microbiology. 2nd edition.
"Green House Gases." The Environmental Literacy Council. 2002. Last Updated May 2007. http://www.enviroliteracy.org/article.php/428.html
Scow, K (2008). Nitrogen Cycle
Dalhgren, R (2007). Topic 3 Lecture on Nutrient Cycling
Green S., and Cresser M., (2008) Nitrogen Cycle Disruption through the Application of De-icing Salts on Upland Highways. Water, Air, and Soil Pollution volumes 188 numbers 1-4:pages 139-153
Richter, A.; Daims, H.; Reigstad, L.; Wanek, W.; Wagner, M.; Schleper,. "Archaeal Nitrification in Hot Springs" American Geophysical Union, Fall Meeting 2006, abstract #B13E-06 http://adsabs.harvard.edu/abs/2006AGUFM.B13E..06R
ScienceDaily (Jun. 13, 2003) "Changing Global Nitrogen Cycle Impacting Human Health, Says Colorado University-led Study" http://www.sciencedaily.com/releases/2003/06/030613074458.htm Site Visited March 21st.
(7)http://toxics.usgs.gov/topics/rem_act/nitrate_remediation.html (8)http://www.umich.edu/~lehnert/denitrification.html
Edited by student of Kate Scow
