Stomach
Introduction to Stomach Niche
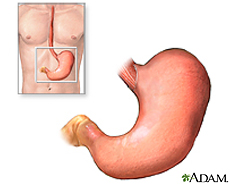
The stomach is an organ located between the esophagus and the small intestine. Food enters through the head of the stomach (cardia) and exits through the tail (pylorus) after digestion. The volume of the stomach increases at meal times and decreases as chyme leaves the stomach and enters the small intestine. The relaxed (empty) stomach has folds of lumen, which expands upon food arrival and almost disappears in a full stomach [11]. Enzymes and acids are secreted in the stomach to break down food molecules, which is stored for gradual energy use.
Animals have one of three main types of diets: Omnivores, Carnivores, or Herbivores. More specifically, human, cat, and cow respectively represent these diets. These three species are categorized by the nutrients that they consume and are distinguished by the unique characteristics of their stomach adapting to these different nutrients.
Omnivores: Human
Special Enviroment
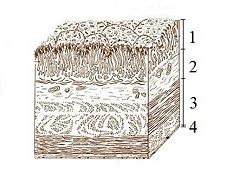
The lumen of the stomach has four distinct layers: 1. Mucosa: an epithelial layer that lines the lumen; 2. Submucosa: a matrix of connective tissue that contains blood vessels and nerves; 3. Muscularis: consists mainly of smooth muscle tissue; 4. Serosa: a thin layer of connective and epithelial tissue external to the muscularis.
The stomach has three specialized cells that are located in the lining of numerous deep pits in the stomach wall. The mucus cells secrete mucus that lubricates the stomach cells, which provides protection from the stomach’s acidic environment. The chief cell secretes an enzyme known as Pepsinogen, which is an inactive form of pepsin that helps to denature proteins from absorbed nutrients inside the stomach. Lastly, the parietal cell secretes hydrochloric acid, which it is responsible for the stomach’s acidic environment [11].
Microbes
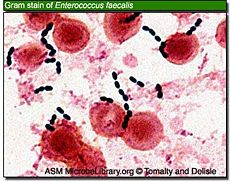
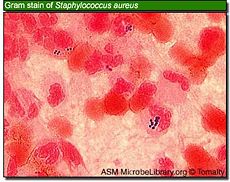
Killing of bacteria requires a pH of less than 2.0, a value which is rarely maintained for any length of time, especially during food intake, which is when most bacteria enter the stomach. Micro-organisms Klebsiella, Salmonella, Shigella flexneri, Proteus, Enterobacter, Enterococcus faecalis, Enterococcus faecium, Staphylococcus epidermidis, Staphylococcus aureus and Candida albicans, did not survive pretreatment at pH 1.0 or 2.0, whereas at pH 4.0, all bacteria tested survived. [22]
Bacteria, however, began to develop resistance to the effects of acid. [23] Acid resistance of bacteria such as E. coli and H. pylori is related to increased buffering capacity within the bacteria and the enhanced production of certain membrane proteins [24] To explain H. pylori colonization of the stomach, as little as 20 min might be sufficient for some bacterial cells to reach the mucosa, and survival may be more likely to occur at slightly higher pHs and in the achlorhydric state [25]. At pH 3.5 total kill is probably not achieved until 80 min, and by this time more than 60% of the stomach contents has emptied, releasing the bacteria into the high pH (>7.0) of the duodenum and allowing further multiplication. [22]
There are three specific conditions that allow bacteria to survive through the harsh acidic conditions of the stomach: bacteria can be protected from low pH by binding to food constituents, [26] usage of antacids or acid blockers would also substantially increase the chances of gastric survival and influence subsequent infection by increasing the stomach pH, [27] and they [babies] produce lower levels of acid in their stomach allowing spores to escape being killed in the stomach [28].
Helicobacter pylori
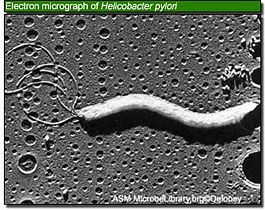
- Significant Discovery of H. pylori
The discovery of Helicobacter pylori as an infectious agent responsible for peptic ulcer disease marked a turning point in the understanding of gastrointestinal microbial ecology and disease. The accepted medical paradigm was that the excess acid causes stomach ulcer by damaging the gastric mucosa, and treatment should be aimed at reducing or neutralizing that acid; due to its low pH environment, no bacteria can survive. However, in 1982, Barry Marshall and J. Robin Warren isolated a new bacterium and showed the relationship between the bacterium and diseases such as gastritis and stomach ulcers [4]. Marshall and Warren cultured the new organism that was similar to Campylobacter in several respects, including curved rod-shaped morphology, optimal growth under microaerophilic conditions, failure to ferment glucose, and G+C content of 34%. The Campylobacter-like organism referred to Campylobacter pylori. Even though C. pylori resembled Campylobacter in many aspects, there were some distinct features: flagellum morphology, fatty acid content, and 16S rRNA sequence. Therefore, C. pylori was transferred to a new genus, Helicobacter, and renamed Helicobacter pylori in 1989. H. pylori was the first member of the new genus and the genus Helicobacter was expanded tremendously and new species are regularly included [2, 3]. Although isolating H. pylori was a significant work, they still did not prove whether the bacteria were the cause of the inflammation. Using Koch’s postulates, Marshall confirmed the connection between H. pylori and gastritis, but since stomach ulcer did not occur, that connection was still unproven. The epidemiological studies later deduce the connection between H. pylori and ulcers [4].
- Morphology and Growth Conditions
H. pylori is an S-shaped bacterium, 0.5 X 5 μm in length, with a tuft of 5 to 7 polar sheathed flagella in one end. The cell is a non-spore-forming, gram-negative bacterium consisting outer and inner membranes that are separated by the periplasm [1, 3]; its morphology is similar to C. jejuni [6]. H. pylori is usually located within the thick mucous layer in close proximity to gastric epithelial cells, which is an acid environment where most bacteria normally can’t survive [1]. It also typically grows under microaerobic conditions at 37°C which is about human body temperature [3]. The nucleoid material and ribosomes exist in the dense cytoplasm and an electron-lucent area is located in the terminal regions. This region and the insertion site located near the flagella is a “polar membrane”; it is an additional electron-dense band below the plasmic membrane while linked to it. ATPase molecules are probably located at this site to generate energy for motility or cell wall synthesis [1].
- Colonization of H. pylori in stomach
There are three essential factors for H. pylori to colonize the gastric mucosa: flagella, urease, and adhesions [2].
1) H. pylori’s unique flagella moves in a screw-like motion, which help the organism to penetrate the mucin layer. The motility of H. pylori is increased when the viscosity of the media is increased in vitro and transverses a methyl glucose solution 10 times more efficiently than Escherichia coli, but the motility is pH dependent and impaired at a pH below 4 [2]. Its flagella are composed of flagellin and are protected by a membranous sheath containing LPS and protein which generates immune response. H. pylori-mediated reduction of mucus synthesis or secretion may also assist the bacterium in accessing to the epithelium [7].
2) Urease is one of the most necessary enzymes in H. pylori pathogenesis; it creates a pH-neutral microenvironment around the bacteria which is necessary for survival in the acidic stomach, pH 1.0-2.0. H. pylori grows best at neutral pH and fails to survive at a pH below 4.0 or above 8.2 in the absence of urea. Urease converts urea from saliva and gastric juices, into bicarbonate and ammonia. Thus, the bacterium is protected by a cloud of acid neutralizing chemicals which is created by urease [2, 5].
3) In both vivo and in vitro, H. pylori adheres to mucin and binds specifically to gastric mucosa epithelial cells [2]. The adherence allows the bacteria to anchor themselves to the epithelial layer, but bacteria that remain sttached to epithelial cells will eventually be swept away as these cells die and are exfoliated. Thus, a proportion of the H. pylori population exists in the nonadherent state. H. pylori must also contend with antibodies and phagocytic cells as the host mounts an immune response [7].
- Transmission
Many researchers think that direct person-to-person contact is the most likely mode of transmission, especially within the family setting. Mother-to-children transmission is known to be more frequent than that of father-to-children transmission; Malaty et al. confirmed that the relative risk of children with H. pylori-positive mothers acquiring infection was 5.3 times that of children whose mothers were H. pylori-negative. In addition to interfamilial transmission, another case-control study found that H. pylori infection may lie outside the family. It is still controversial whether the transmission of the bacterium is gastro-oral, oral-oral, or fecal-oral route. Today, there are many public health measures based on epidemiological data have been extremely successful in preventing the spread of pathogenic agents. Although those measures are successful, the route of transmission of H. pylori should be clarified [8].
- Metabolism
Helicobacter pylori uses glucose as the main source of energy, and the energy is generated through pentose phosphate pathway. The step of metabolic activation by phosphorylation is important in the uptake of saccharides by microorganisms. Covalent attachment of phosphate groups to sugars contribute that it makes the covalent attachment more impermanent to the cell envelope for the intracellular retention of activated saccharides. Phosphorylation of glucose in bacteria is activated by three different kinds of enzymes: hexokinases, glucokinases, and the E-III enzymes of the glucose phosphotransferase system. Pentose phosphate pathway includes both the oxidative and non-oxidative phases; oxidative when producing NADPH and non-oxidative when synthesizing pentose sugars [17].
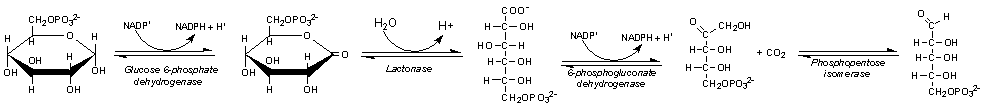
- Disease & Treatments
In human stomach, H. pylori infection can cause diseases such as gastric ulcers and gastritis that can lead to gastric cancer and gastric MALT lymphoma.
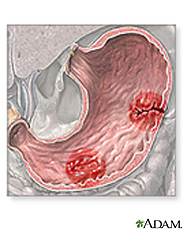
1) Gastric Ulcer
Gastric ulcer is defined as mucosal erosions equal to or greater than 0.5 cm in stomach. As H. pylori lands on the protective mucous lining of the stomach, the infection causes weakening of the protective mucous coating. This allows H. pylori and stomach acid to get into the sensitive mucous lining beneath, causing irritation and formation of ulcer. Symptoms are abdominal pain and ingestion, nausea, vomiting, fatigue, and unintended loss of weight. Gastric ulcer is treated by eradication of the bacteria that causes infection by using medicines: Pepcid, Axid, Prilosec, or Nexium. These medicines are used in order to suppress acid and kill the bacteria in stomach. [17, 18]
2) Gastric Cancer
H. pylori infection can also activate the innate and acquired immune responses, as well as effecting changes in the hormonal milieu and acid secretory physiology of the stomach. These changes combine to alter the balance of apoptosis and proliferation, and promote the loss of acid-secreting parietal cells. The increase in apoptosis occurs in large part through activation and increased signaling through the Fas/Fas L pathway. Parietal cell loss is compensated by expansion of a less differentiated precursor lineage that is predisposed to give rise to cancer. The symptoms of the early stages of gastric cancer are: heartburn, stomach discomfort, nausea, and loss of appetite. In advanced stages of gastric cancer, the symptoms occur: vomiting, abdominal pain, unintentional weight loss, and jaundice. Gastric Cancer is treated by surgery, which leades to removing the part of the stomach that has cancer nearby lymph nodes, tissues, and spleen. Or a surgery called "Total gastrectomy" removes the entire stomach and connects esophagus to the small intestines in order for the patient to ingest food. Other types of therapies used are: Chemotherapy, Radiation therapy, and chemoradiation. Chemotherapy uses drugs to prevent the growth of cancer and it stops them from dividing in stomach. Radiation therapy treats x-rays and radiation to kill cancer cell, and this also prevents the growth of cancer cell. Chemoradiation is a treatment that uses both Chemotherapy and Radiation therapy. Rather than lowering the pH level in stomach, the cancer cells are treated with more powerful tools. [17, 18]
3) MALT(Mmucosa Associated Lymphoid Tissue) Lymphoma
H. pylori infection can cause inflammation of the lining of the stomach. By normal immunal reaction of the stomach, the inflamation developes MALT-type lymphoid tissues which triggers continuous stimulation of the lymphocytes to replicate and increase in number. However, in a small minority of people, this results in a mistake within the genetic material of a lymphoid cell and continuation of this faulty cell line leads to the development of a lymphoma. The symptoms of MALT Lymphoma are: abdominal pain, fatigue, and fever. MALT Lymphomas can be treated with antibiotics to eliminate H. pylori or stopping them from diving in stomach. The condition in stomach becomes more acidic as the antibiotics were used. [17, 18]
Clostridium botulinum
Clostridium botulinum is a toxin producing bacteria that upon ingestion causes a disease known as botulism. This bacterium is mostly found in water and soil. The Clostridium botulinum toxin inhibits proper functioning of the nerves and paralyzes muscles. Early symptoms of botulism, starts from the numbness of the toes and in severe cases migrates up the lungs, which can cause respiratory failure for an individual.
C. botulinum can exist in two forms, vegetative cells and spores. Oxygen is able to kill the vegetative cells of C. botulinum. However, in its spore formation, it is able to be resistant to oxygen and moderate heat. When circumstances are neutral, the spores are then able to germinate to reform the vegetative form and produce toxin. Botulinum toxin can be very potent and minute amounts are needed to cause severe disease.
- Transmission: Botulism is mostly associated with food consumption.
- Prevention: C. botulinum spores are highly heat resistant. Temperature must exceed 121 C (250 F) to kill off spores and 85 C (185 F) to kill off vegetative cells.
- Symptoms: Symptoms include blurred vision dry mouth, constipation, abdominal pains, drooping eyelids, slurred speech, difficulty swallowing, and muscle weakness, which may ultimately lead to paralysis. [33]
- Baby botulism: Infants under 1 year of age consume the spores of the botulinum bacteria from outside environment such as soil, dust. The most common food source associated with infant botulism is honey. When an infant baby consumes the spores of the botulinum bacteria, it germinates to its vegetative cell form and releases toxin. The baby’s stomach pH is not as strong as an adult and therefore spores are able to escape being killed in the stomach. The intestines of the infants also lack the proper number of normal bacterial flora to prevent the growth of the vegetative C. botulinum cells. [28]
Microbes that survive the stomach's acidity
1. Noroviruses (formerly called “Norwalk-like Viruses”) are the prototype of caliciviruses. They are single-stranded, non-enveloped RNA-viruses and measure 28–35 nm. [19]
- Infection site: Small intestine.
- Disease: Gasteroenteritis also known as the "stomach flu" [20]
- Symptoms: Symptoms include vomiting, diarrhea nausea, abdominal cramps, headache, myalgia, chills and fever. Symptoms typically last between one and three days and recovery is usually rapid. [21]
- Transmission: Norovirus may be acquired through consuming contaminated food or water, or more commonly from an infected person via the oral route or by aerosol spread, particularly when a case vomits. Semi-closed communities such as hotels, cruise ships and hospitals are particularly prone to large outbreaks. [21]

- Treatment: None. Currently, there is no medication or vaccine for norovirus and it cannot be treated with antibiotics. By drinking fluids, such as juice or water, people can reduce their chance of becoming dehydrated. [20]
2. Rotavirus is the leading cause of diarrhea hospitalization among children worldwide.
- Infection: Cells of the small intestine.
- Symptoms: Rotavirus infection symptoms include: fever, nausea, vomiting, abdominal cramps, watery diarrhea, cough, and runny nose.
- Transmission: The virus passes in the stool of infected persons before and after they have symptoms of the illness. Rotavirus infection outbreaks are most common during the winter and spring months.
- Prevention: Rotavirus vaccine is included in the lineup of routine immunizations given to all infants. The immunization is given by mouth at around 2, 4, and 6 months of age. In general, frequent hand washing is the best tool to limit the spread of rotavirus infection.
- Treatment: Dehydration must be treated by bring the body’s fluid and salt levels back to normal. A child can be treated in a hospital with intravenous (IV) fluids or treated at home by eating normally with more fluids. [34]
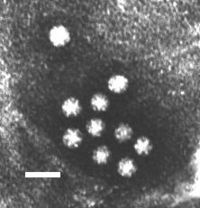
3. Astrovirus belongs to the Astroviridae family given this name due to its star-shaped configuration. It is a single-stranded RNA virus with 6.8-7.3kb in genome length.
- Infection: Lamina propria of small intestine
- Symptoms: Astrovirus infection symptoms include: diarrhea, nausea, vomiting, fever, and abdominal pains. These symptoms are expected to last three to four days.
- Transmission: The main source of Astrovirus’ transmission is through contaminated food and water.
- Prevention: There are currently no vaccine or anti-viral treatment against Astrovirus.
- Treatment: No treatment is necessary as the symptoms disappear themselves after a short given time. [35]
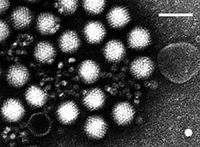
4. Adenovirus is an infectious agent that disrupts tissue cultures of adenoidectomy specimens from children. Adenovirus is perhaps the best known cause of acute respiratory infection.
- Symptoms: Depending on which part of the body is affected, the symptoms of Adenovirus infection vary.
- - Respiratory: Symptoms of infection in the respiratory tracts include: fever, sore throat, congested nose, runny nose, and cough
- - Gastroenteritis: Symptoms of infection in the stomach and intestines include: watery diarrhea, vomiting, headache, fever, and abdominal cramps.
- - Urinary: Symptoms of infection in the urinary tracts include: burning, pain, and blood in urine.
- - Eye infection: Symptoms of infection in the eye include: red eyes, discharge, tearing, and pain
- Transmission: Adenovirus is highly contagious and can be transmitted through respiratory secretion by coughing or sneezing, and ingested through contaminated food and water. Symptoms may appear as early as two days from exposure up to two weeks.
- Prevention: There are currently no vaccine or anti-viral treatment against Adenovirus. To reduce the risk transmission, wash hands and clean surfaces frequently.
- Treatment: There are currently no treatments for Adenovirus. One should eat normally and drink fluid to prevent dehydration.
Carnivores: Cats
Special Environment
The ability of the carnivore stomach to secrete hydrochloric acid is exceptional. Carnivores are able to keep their gastric pH down around 1-2 even with food present. This is necessary to facilitate protein breakdown and to kill the abundant dangerous bacteria often found in decaying flesh foods [9].
Microbes
Bacteria such as Salmonella, Shigella, Escherichia coli (E.Coli) O157:H7 and other food borne pathogens are skillfully handled by the extended time in the strongly acidic environment of the stomach. The extremely low pH characteristic of the stomach is mandatory in order to deal with the bacteria that enter by consuming raw meat of the prey.[10]
Herbivores: Cows
Special Environment
Cow is a ruminant which is a foregut fementor with a four-chambered stomach. Four chambers are rumen, reticulum, omasum, and abomasum and among them, abomasum has the most similar function to the stomach of a man [12]. Its pH is normally around 6.0 but because its walls secrete enzymes and hydrochloric acid, the pH is able to be lowered to about 2.5. [13]. To store large quantities of food, there is rumen which is the largest compartment [13]. The environment within the rumen is anaerobic, 39C, with a pH between 6.0 and 7.0. [12]. Due to its high and constant pH, the rumen is the perfect place for the bacteria to live.
Microbes
Rumen bacteria are classified into 4groups; fiber digesters, starch and sugar digesters, lactate using bacteria, and hydrogen-using bacteria [14]. They cooperate together.
Megasphaera elsdenii
It is an anaerobic bacterium which belongs to lactate fermenting species [15]. Megasphaera elsdenii grows using lactic acid and as a result the rumen is cleaned up. This helps to raise the pH of rumen which is good for the growth of the acid-intolerant bacteria [14].
Streptococcus bovis
Streptococcus bovis is one of the most acid-tolerant bacteria among ruminal bacteria. It produces lactic acid when ruminants ingest high-starch diets and pH is low [16]. As mentioned above, Megasphaera elsdenii uses lactic acid which is from Streptococcus bovis to grow and consequently pH is raised. However, because of its quick raising of pH, rumen acidosis can be caused.
Current Research
1. Stomach Cancer Risk Reduced by Bacteria Removal, 2008
According to the researchers, Dr. Mototsugu Kato and Dr. Masahiro Asaka, from Hokkaido University Graduate School of Medicine, Japan, removal of Helicobacter Pylori bacteria in stomach can greatly reduce the redevelopment of gastric cancer. The team tested “the possible prophylactic effect of H. pylori eradication on the development of metachronous stomach cancer after surgery to remove early gastric cancer.” (Metachronous cancer develops to remove the initial cancer after the surgery and it develops at a new site in stomach.) Among a randomized 544 patients, the half of the patients received a H. pylori eradication and the other half received the standard care but no treatment. As a result, 9 patients in the eradication group and 24 in the standard care group had developed metachronous gastric cancer. This concludes that the risk of developing metachronous cancer was reduced by two thirds in the eradication group. [29]
2. Microbes are adapt to inner space, 2006
Martin J. Blaser researched in adapting of microbes to our bodies. The human gastric bacterium Helicobacter pylori get cholesterol which is from its host and change the molecule enzymatically to protect itself from the immune system. The finding reveals yet another strategy used by successful microbial colonizers to evade immunity. [30]
3. MALT lymphoma in patients with autoimmune diseases: a comparative analysis of characteristics and clinical course, 2007
Since 1997, 158 patients were diagnosed and treated for MALT lymphoma and they have undergone serological assessment for autoimmune disease (AD). Out of total 158 patients, 61 patients were diagnosed with an AD. Lymphomas in these patients showed a very low response rate to H. pylori eradication therapy in the case of gastric lymphomas. This concludes that the patients with AD develop MALT lymphoma significantly earlier in life compare to the patients without AD. [31]
4. Genome evolution in host-adapted bacteria, 2005
Stephan C. Schuster and his lab researched in identifying genes unique to Wolinella succinogenes, Campylobacter jejuni, and Helicobacter pylori. W. succinogenes is a bacterium isolated from cow ruminal fluid and Schuster shows that W. succinogenes has a genome 30% larger than C. jejuni and H. pylori. By differential approach, Schuster can identify genes unique to each organisms and may confer host specificity to each organisms. On the other hand, by studying the genes shared by these three organisms, Schuster hopes to identify essential molecular mechanisms used by symbiotic, commensal or pathogenic bacteria to maintain themselves in a vertebrate host environment. [32]
Summary
References
1. O’Rourke, Jani and Bode, Gunter. “Morphology and Ultrastructure.” Helicobacter pylori: Physiology and Genetics, 2001. 6: 53-67.
2. Anderson, L. P. and Wadstrom, Torkel. “Basic Bacteriology and Culture.” Helicobacter pylori: Physiology and Genetics, 2001. 4: 27-38.
3. Solnick, Jay V. and Vandamme, Peter. “Taxonomy of the Helicobacter Genus.” Helicobacter pylori: Physiology and Genetics, 2001. 5: 39-51.
4. Lynch, Nancy A.. “Helicobacter pylori” and Ulcers: a Paradigm Revised.
5. Sachs, George, Scott, David R., Weeks, David L., Rektorscheck, Marina, and Melchers, Klaus. “Regulation of Urease for Acid Habitation.” Helicobacter pylori: Physiology and Genetics, 2001. 25: 277-291.
6. Dubois, Andre. “Spiral Bacteria in the Human Stomach: The Gastric Helicobacters.” Emerging Infectious Diseases, 1995. Vol. 1, No. 3.
7. Testerman, Traci L., McGee, David J., and Mobley, Harry L.. “Adherence and Colonization.” Helicobacter pylori: Physiology and Genetics, 2001. 34: 381-417.
8. Mitchell, Hazel M.. “Epidemiology of Infection.” Helicobacter pylori: Physiology and Genetics, 2001. 2: 7-18.
9. Mills, Milton R. “The Comparative Anatomy of Eating” VegSource Interactive Inc. 26 March 1996.
10. Simpson, JW, and Else, RW. (1991) Digestive Disease in the Dog and Cat. Blackwell Scientific Publications.
11. Martini, Frederic, Michael Timmons, Robert Tallitsch. Human Anatomy. 5th ed. San Francisco: Pearson Education Inc. 2006. 662-663.
12. Dehority, B. "Gastrointestinal tracts of herbivores, particularly the ruminant: Anatomy, physiology and microbial digestion of plants" Journal of applied animal research, 21.2 (2002) 145-160
13. Umphrey, J.E., and C. R. Staples. "General Anatomy of the Ruminant Digestive System" University of Florida, Institute of Food and Agricultural Sciences (1992)
14. Beth de Ondarza, Mary “Rumen Microbiology” Milkproduction.com (2000)
15. Marounek, M., K. Fliegrova, and S. Bartos "Metabolism and some characteristics of ruminal strains of Megasphaera elsdenii" Applied and Environmental Microbiology (1989) 1570-1573
16. Asanuma, N., and T. Hino "Regulation of fermentation in a ruminal bacterium, Streptococcus bovis, with special reference to rumen acidosis" Animal Science Journal 73 (2002) 313-325
17. Goodwin, C. Steward, and Worsley, BW. (1993) Helicobacter pylori. Biology and Clinical Practice, 115-141
18. Hunt, R., Tytgat, G. (2002) Helicobacter pylori. Basic Mechanisms to Clinical cure 2002, 171-177
19. Leuenberger, Samuel, Marc-Alain Widdowsonb, Jonas Feilchenfeldta, Richard Eggerc, and Rolf A. Streuli. “Norovirus outbreak in a district general hospital” Swiss Med Wkly. 137 (127) 57
20. Lindesmith L, C Moe , and S Marionneau. "Human susceptibility and resistance to Norwalk virus infection". Nat. Med. 5 (2003) 548–53
21. Cowden, J., A Smith-Palmer, and C. Kilpatrick. “Norovirus infection in Scotland” Scieh Weekly Report. 38 (2004) 118
22. Zhu, H., C. Hart, D. Sales, and N. Roberts. “Bacteria killing in gastric juice – effect of pH and pepsin on Escherichia coli and Helicobacter pylori”
23. Small, P., D. Blankenhorn, D. Welty, E. Zinser, and J. Slaczewski. “Acid and base resistance in E. coli and Shigella flexneri: role of rpoS and growth pH” J Bacteriol 176 (1994) 1729–1737
24. Booth, I. “Regulation of cytoplasmic pH in bacteria” Microbiol Rev 49 (1985) 359–378
25. Balan, K., A. Jones, N. Roberts, J. Pearson, M. Critchley and S. Jenkins. “The effects of Helicobacter pylori colonization on gastric function” Am J Gastroenterol 91 (1996) 1400–1406
26. Rosina, A. “Human intestinal flora in health and disease” PhD thesis, University of Liverpool (1982)
27. Koo, Jaheon, Douglas L. Marshall, and Angelo DePaola. “Antacid Increases Survival of Vibrio Vulnificus and Vibrio vulnificus Phage in a Gastrointestinal Model” Applied and Environmental Microbiology. 67.7 (2001) 2895-2902
28. Chamberlain, Neil. “What is Botulism (Clostridium botulinum toxin)?” 2005. A.T. Still University of Health Sciences/Kirksville College of Osteopathic Medicine. 28 Aug. 2008 <http://www.kcom.edu/faculty/chamberlain/bioterror/botulism.htm>
29. Anonymous. "Stomach cancer risk reduced by bacteria removal" 2008. Cancer Intelligence. 08 Jan. 2008 <http://www.ecancermedicalscience.com/news-insider-news.asp?itemId=252>
30. Blaser, Martin J. “Microbes adapt to inner space” Nature Medicine 12 (2006) 994-996
31. Wöhrer, S., M Troch, B Streubel, J Zwerina, C Skrabs, M Formanek, W Hauff, M Hoffmann8, L Müllauer, A Chott and M Raderer. "MALT lymphoma in patients with autoimmune diseases: a comparative analysis of characteristics and clinical course" 2007. Leukemia. 07 Jun. 2007 <http://www.nature.com/leu/journal/v21/n8/abs/2404782a.html>
32. Schuster, Stephan C. "Genome evolution in host-adapted bacteria." 2005 Penn State University 27 Aug. 2008. <http://www.bmb.psu.edu/faculty/schuster/schuster.html>
33. Arnon, Stephen, Robert Schechter, Susan Maslanka, Nicholas Jewell, and Charles Hatheway, “Human Botulism Immune Globulin for the Treatment of Infant Botulism” The New England Journal of Medicine. 354.5 (2006) 462-471
34. Parashar, Umesh, Christopher Gibson, Joseph Bresee, and Roger Glass. “Rotavirus and Severe Childhood Diarrhea” Center for Disease Control and Prevention. 12.2 (2006) 304-306
35. Papaventsis, Dimitrios, Winifred Dove, Nigel Cunliffe, Osamu Nakagomi, Patrice Combe, Pierre Grosjean, and C. Anthony Hart. “Human Astrovirus Gastroenteritis in Children, Madagascar” Center for Disease Control and Prevention. 14.5 (2008) 844-846
Edited by [Sohyun Ahn, Byoung Cho, Seong Kang, Geon Woo Kim, Yoo Na Kim, and Sukwoo Kong] students of Rachel Larsen
