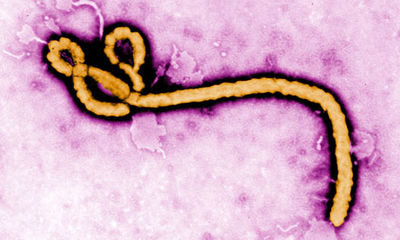
Ebola Transmission
Introduction
The Ebola virus is a relatively recently described, severe disease-causing pathogen that poses a huge threat to human health mostly within central Africa. This is, in part, due to its high mortality4 and lack of affordable treatment options. Ebola is considered a Biosafety level 4 (BSL-4) agent; classifying it among the most threatening pathogens that exist in the world today. Agents within this category pose severe threats to human health and can be fatal due to the lack of available vaccines. There are five known Ebola species within the family Filoviridae, four of them endemic to Africa[6], and all of the species within the family cause varying degrees of viral hemorrhagic fever illnesses.[4]
Virus Structure and Infection
The Ebola virus has an RNA genome that codes for at least seven proteins and is enclosed in a nucleocapsid[3]. Four of the virus’ proteins are thought to comprise the capsid. The capsid allows the transcription and the translation of the viral genome and is therefore the principle player in the virus’ pathogenicity[4]. In addition, the virus has been shown to impair the immune function of the body by way of its infection of the phagocytic cells. The nucleocapsid and viral proteins move to new sites of infection and form themselves into virons where they can then go on to infect around the host body.
The virus forms many long rods and is much longer than it is wide and is often photographed in a hooked or curved form. The virus is around 80nm in diameter and can be of varying lengths ranging from 600-1400nm[1]. Ebola does not produce its own cell membrane and instead steals some of its host membrane to survive. [4][2]
In recent study it has been indicated that the Ebola virus has developed several customized ways to enter host cells depending on the virus' size and what kind of cell the virus wants to gain access to[1]. However, it seems in most cases the virus prefers to use macropincytosis, a form of endocytosis, which allows the cell to steal some of the hosts' membrane as it leaves. It seems that mononuclear phagocytes, hepatocytes, and endothelial cells are the cells that are preferred targets of the infection.
Containment and Transmission
Containment
Due to its classification as a level four safety agent, studying
Ebola in the lab is no small feat. The virus is not only well adapted
to infect its hosts, but also excels at spreading from host to host.
Scientists who choose to work with
Ebola must use the utmost caution to prevent the virus' ability to infect
them while they conduct their experiments. Full hazard suits must be
worn at all times and each suit is throughly examined before entering
a containment space.
Transmission
In the past it was thought that the virus only infected and caused death
in humans and non-human primates mostly in the central African region.
The disease has been known to spread from human to human by way of
contaminated bodily fluids which happens to be more common in developing nations.
Additionally, the main reservoirs of infection was
thought to be the fruit bat[6]. But, in recent years, as more
study is being conducted on the virus, it seems that there is more to its
complex interactions among multiple species. It was found that Ebola can, and does, infect pigs and can pass on to nonhuman primates.[6] This is an early sign that pigs may play a role in human infection processes. However, in the pig the virus seems to be airborne, a dramatic change from what was previously known about the disease. This has far reaching consequences for treatment and containment.[6]
Treatments
The best treatment for Ebola came about several years ago in the form of a vaccination. The only downside to this approach is that Ebola outbreaks are random and occur in undefined locations during the span of one year. Many individuals in Africa do not receive vaccines upon birth and therefore are not protected against the virus. In addition, the vaccination series lasts six months while the disease can often be fatal in less than half of that time.[6] These two factors taken together make coming up with a lasting and final solution to the Ebola problem a difficult task for scientists.
Ebola vaccine.jpg
The most attractive solution at this point in time seems to be the formation of a nonreplicating subunit vaccine. This is the ideal candidate because the vaccine is administered in one dosage and has been shown to be effective in preventing infection in up to 80% of exposed test animals. This is a huge advancement and turn around from the diseases' unusually high mortality rate. This particular treatment garners extra support due to the fact that it can be stockpiled and stay effective for long periods of time. This indicates that the vaccine can be pulled out when the first signs of the disease is seen in a population and thus save many lives.[5]
Conclusion
In today's world Ebola is still a huge problem that faces many developing nations within Africa. In 2012 alone there were 5 outbreaks of Hemorrhagic Fevers around the world and 3 of them were due to the Ebola virus.7 One of the factors that makes this disease so clinically challenging is the fact that it is indistinguishable from other agents that cause alternative forms of hemorrhagic fevers[3]. Because some of these diseases are treatable doctors in at risk areas must depend on detailed patient history accounts which can be a roadblock due to language barriers.
The best way to move forward from the pain caused by all of the species of Ebola is to continue research on promising single-step vaccines and begin to move them into human trial stages. The fact that some strains of Ebola can have up to an 88%[3][5] mortality rate should be enough of an incentive to push research forward. In addition, the world as a whole needs to start to get serious about ways to make affordable, life-saving vaccinations available to those who need them most.
References
3. "Ebola virus: from discovery to vaccine". Nature Reviews Immunology 3. 2003. p. 667- 85.
Edited by (Victoria Rose Gawlik), a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.